Steroids for symptom control in infectious mononucleosis
- PMID: 26558642
- PMCID: PMC7047551
- DOI: 10.1002/14651858.CD004402.pub3
Steroids for symptom control in infectious mononucleosis
Abstract
Background: Infectious mononucleosis, also known as glandular fever or the kissing disease, is a benign lymphoproliferative disorder. It is a viral infection caused by the Epstein-Barr virus (EBV), a ubiquitous herpes virus that is found in all human societies and cultures. Epidemiological studies show that over 95% of adults worldwide have been infected with EBV. Most cases of symptomatic infectious mononucleosis occur between the ages of 15 and 24 years. It is transmitted through close contact with an EBV shedder, contact with infected saliva or, less commonly, through sexual contact, blood transfusions or by sharing utensils; however, transmission actually occurs less than 10% of the time. Precautions are not needed to prevent transmission because of the high percentage of seropositivity for EBV. Infectious mononucleosis is self-limiting and typically lasts for two to three weeks. Nevertheless, symptoms can last for weeks and occasionally months.Symptoms include fever, lymphadenopathy, pharyngitis, hepatosplenomegaly and fatigue. Symptom relief and rest are commonly recommended treatments. Steroids have been used for their anti-inflammatory effects, but there are no universal criteria for their use.
Objectives: The objectives of the review were to determine the efficacy and safety of steroid therapy versus placebo, usual care or different drug therapies for symptom control in infectious mononucleosis.
Search methods: For this 2015 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 7), which includes the Cochrane Acute Respiratory Infections Group's Specialised Register; MEDLINE (January 1966 to August 2015) and EMBASE (January 1974 to August 2015). We also searched trials registries, however we did not identify any new relevant completed or ongoing trials for inclusion. We combined the MEDLINE search with the Cochrane search strategy for identifying randomised controlled trials (RCTs). We adapted the search terms when searching EMBASE.
Selection criteria: RCTs comparing the effectiveness of steroids with placebo, usual care, or other interventions for symptom control for people with documented infectious mononucleosis.
Data collection and analysis: We used the standard methodological procedures expected by Cochrane.
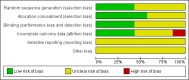
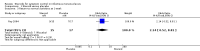
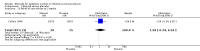
Main results: For this 2015 update, we did not identify any new RCTs for inclusion. The previous version of the review included seven trials with a total of 362 participants. Four trials compared the effectiveness of a steroid to placebo for short-term symptom control in glandular fever, one to aspirin, and two trials explored the effects of steroids in conjunction with an antiviral. Heterogeneity between trials prevented a combined analysis.Trials under-reported methodological design features. Three trials did not adequately describe sequence generation for randomisation. Four trials provided adequate details of allocation concealment. All trials were double-blind but four were not specific as to who was blinded. Loss to follow-up was under-reported in four trials, making it difficult to exclude attrition bias. The risk of selective reporting in the included trials was unclear.Across the trials, no benefit was found in 8/10 assessments of health improvement. Two trials found benefit of steroid therapy over placebo in reducing sore throat at 12 hours (eight-day course odds ratio (OR) 21.00, 95% confidence interval (CI) 1.94 to 227.20; one-dose OR 4.20, 95% CI 1.08 to 16.32), but the benefit was not maintained.In combination with an antiviral drug, participants in the steroid group had less pharyngeal discomfort between days two to four (OR 0.31, 95% CI 0.09 to 1.08) compared to placebo. Across the trials the effects on other common symptoms were less clear. Two trials set out to measure safety; they documented no major adverse effects. In two other trials adverse events were reported, including respiratory distress and acute onset of diabetes. However, the association of the events with the steroid is not definite.
Authors' conclusions: There is insufficient evidence to the efficacy of steroids for symptom control in infectious mononucleosis. There is a lack of research on the side effects and long-term complications.
Conflict of interest statement
Emtithal Rezk: none known. Yazan H Nofal: none known. Ammar Hamzeh: none known. Mohammad A AlKheder: none known. Muhammad F Al Hammad: none known. Muhammed F Aboujaib: none known.
Figures































Update of
-
Steroids for symptom control in infectious mononucleosis.Cochrane Database Syst Rev. 2006 Jul 19;(3):CD004402. doi: 10.1002/14651858.CD004402.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2015 Nov 08;(11):CD004402. doi: 10.1002/14651858.CD004402.pub3. PMID: 16856045 Updated.
Comment in
-
No new evidence to support the routine use of steroids in the treatment of infectious mononucleosis.Evid Based Med. 2016 Jun;21(3):103. doi: 10.1136/ebmed-2016-110404. Epub 2016 Apr 20. Evid Based Med. 2016. PMID: 27099076 No abstract available.
References
References to studies included in this review
Bolden 1972 {published data only}
Collins 1984 {published data only}
-
- Collins M, Fleisher G, Kreisberg J, Fager S. Role of steroids in the treatment of infectious mononucleosis in the ambulatory college student. Journal of American College Health 1984;33(3):101‐5. - PubMed
Klein 1969 {published data only}
-
- Klein E, Cochran JF, Buck RL. The effects of short‐term corticosteroid therapy on the symptoms of infectious mononucleosis pharyngotonsillitis: a double‐blind study. Journal of American College Health Association 1969;17(5):446‐52. - PubMed
Prout 1966 {published data only}
-
- Prout C, Dalrymple W. A double‐blind study of eighty‐two cases of infectious mononucleosis treated with corticosteroids. Journal of American College Health Association 1966;15(1):62‐6. - PubMed
Roy 2004 {published data only}
-
- Roy M, Bailey B, Amre DK, Girodias J‐B, Bussieres J‐F, Gaudreault P. Dexamethasone for the treatment of sore throat in children with suspected infectious mononucleosis. Archives of Pediatrics and Adolescent Medicine 2004;158(3):250‐4. - PubMed
Simon 2003 {published data only}
-
- Simon MW, Deeter RG, Shahan B. The effect of valacyclovir and prednisolone in reducing symptoms of EBV illness in children: a double‐blind, placebo‐controlled trial. International Pediatrics 2003;18(3):164‐9.
Tynell 1996 {published data only}
-
- Tynell E, Aurelius E, Brandell A, Julander I, Wood M, Yao Q‐Y, et al. Acyclovir and prednisolone treatment of acute infectious mononucleosis: a multicenter, double‐blind, placebo‐controlled study. Journal of Infectious Diseases 1996;174:324‐31. - PubMed
References to studies excluded from this review
Andersson 1988a {published data only}
-
- Andersson J, Ernberg I. Management of Epstein‐Barr virus infections. American Journal of Medicine 1988;85:107‐15. - PubMed
Antila 1962 {published data only}
-
- Antila V, Makela TE, Klemola E. Corticotropin in the treatment of infectious mononucleosis. Acta Medica Scandinavica 1962;171:345‐8. - PubMed
Bender 1967 {published data only}
-
- Bender CE. The value of corticosteroids in the treatment of infectious mononucleosis. JAMA 1967;199:97‐9. - PubMed
Brandfonbrener 1986 {published data only}
-
- Brandfonbrener A, Epstein A, Wu S, Phair J. Corticosteroid therapy in Epstein‐Barr Virus infection. Archives of Internal Medicine 1986;146:337‐9. - PubMed
Breen 1965 {published data only}
-
- Breen GE, Talukdar PK. Corticosteroids in the acute infections. Lancet 1965;1(7377):158‐60. - PubMed
Evans 1960 {published data only}
-
- Evans AS. Infectious mononucleosis in university of Wisconsin students. American Journal of Hygiene 1960;71:342‐62. - PubMed
Gordon 1968 {published data only}
-
- Gordon MP. Corticosteroid treatment of infectious mononucleosis in a military population. Military Medicine 1968;133(4):303‐5. - PubMed
Schumacher 1963 {published data only}
-
- Schumacher HR, Jacobson WA, Bemiller CR. Treatment of infectious mononucleosis. Annals of Internal Medicine 1963;58:217‐28. - PubMed
Simonsen 1996 {published data only}
-
- Simonsen E, Christensen K. Mononucleosis Infectiosa, a 5‐year material with special reference to the effect of prednisolone treatment. Acta Medica Scandinavica 1996;180:729‐35. - PubMed
Additional references
AAFP 2000
-
- American Academy of Family Physicians. Infectious mononucleosis. http://www.kidshealth.org/PageManager.jsp?dn=familydoctor&article_se... (accessed 2000). . ..
Aronson 2014
-
- Aronson MD, Auwaerter PG. Infectious mononucleosis in adults and adolescents. http://www.uptodate.com/contents/infectious‐mononucleosis‐in‐adults‐and‐... 2014.
Auwaerter 1999
-
- Auwaerter PG. Infectious mononucleosis in middle age. JAMA 1999;281(5):454‐9. - PubMed
Bender 1953
-
- Bender CE, Houghton BE. Treatment of infectious mononucleosis with corticotropin. Northwest Medical Journal 1953;52:922. - PubMed
Berger 2003
Brown 2001
-
- Brown B. Infectious mononucleosis. In: Youngson R editor(s). The Royal Society of Medicine Health Encyclopaedia. 2nd Edition. Bloomsbury Publishing Plc, 2001.
Burton 2000
-
- Burton J. GP's glandular fever strategy produces dramatic results. Pulse 2000;February:38.
Candy 2002
Candy 2005
-
- Candy B, Chalder T, Clear AJ, Wessely S, Hotopf M. What advice do patients with infectious mononucleosis report being given by their general practitioner?. Journal of Psychosomatic Research 2005;58:435‐7. - PubMed
Cohen 2001
-
- Cohen JI. Epstein‐Barr virus infections, including infectious mononucleosis. In: Braunwald E, et al. editor(s). Harrison's Principles of Internal Medicine. 15th Edition. McGraw‐Hill Companies, 2001.
Creditor 1959
-
- Creditor MC, McCurdy HW. Severe infectious mononucleosis treated with prednisolone. Annals of Internal Medicine 1959;50:218. - PubMed
Cunha 2014
-
- Cunha BA. Infectious Mononucleosis. Medscape 2014. [http://emedicine.medscape.com/article/222040‐overview]
Doran 1953
-
- Doran JK, Wiesberger AS. The use of ACTH in infectious mononucleosis. Annals of Internal Medicine 1953;38:1058‐62. - PubMed
Fiese 1953
-
- Fiese MJ. Guillain‐Barré syndrome in infectious mononucleosis; report of a case with recovery following administration of cortisone. Archives of Internal Medicine 1953;92(3):438‐41. - PubMed
Frenkel 1956
-
- Frenkel EP, Shiver CB, Berg P, Caris TN. Meningoencephalitis in infectious mononucleosis; report of a case treated with cortisone. Journal of the American Medical Association 1956;162:885. - PubMed
Ganzel 1996
-
- Ganzel TM, Goldman JL, Padhya TA. Otolaryngologic clinical patterns in pediatric infectious mononucleosis. American Journal of Otolaryngology 1996;17(6):397‐400. - PubMed
Handler 1979
-
- Handler SD, Warren WS. Peritonsillar abscess: a complication of corticosteroid treatment in infectious mononucleosis. International Journal of Pediatric Otorhinolaryngology 1979;1:265‐8. - PubMed
Hellwig 2013
-
- Hellwig T, Jude K, Meyer B. Management options for infectious mononucleosis. US Pharmacist 2013;38(5):38‐41. [http://www.uspharmacist.com/content/d/feature/c/40888/]
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. Chichester: Wiley‐Blackwell. [www.cochrane‐handbook.org]
Jenson 2000
-
- Jenson HB. Acute complications of Epstein‐Barr virus infectious mononucleosis. Current Opinion in Pediatrics 2000;12:263‐8. - PubMed
Johnsen 1981
-
- Johnsen T. Infectious mononucleosis and peritonsillar abscess. Journal of Laryngology and Otology 1981;95:873‐6. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Luzuriaga 2010
-
- Luzuriaga K, Sullivan JL. Infectious mononucleosis. New England Journal of Medicine 2010;362(21):1993‐2000. - PubMed
Maki 1982
-
- Maki DG, Reich RM. Infectious mononucleosis in the athlete. American Journal of Sports Medicine 1982;10:162‐73. - PubMed
Mandel 1955
-
- Mandel W, Marilley RJ, Gaines LM. Corticotropin in severe anginose infectious mononucleosis. Journal of the American Medical Association 1955;158:1021. - PubMed
Mason 1958
-
- Mason WR, Adams EK. Infectious mononucleosis, an analysis of 100 cases. American Journal of Medical Science 1958;236:447‐59. - PubMed
McGowan 1992
-
- McGowan JE Jr, Chesney PJ, Crossley KB, LaForce FM. Guidelines for the use of systemic glucocorticosteroids in the management of selected infections. Working group on steroid use. Antimicrobial Agents Committee, Infectious Diseases Society of America. Journal of Infectious Diseases 1992;165:1‐13. - PubMed
Papesch 2001
-
- Papesch M, Watkins R. Epstein‐Barr virus infectious mononucleosis. Clinical Otolaryngology 2001;26(1):3‐8. - PubMed
Portman 1984
-
- Portman M, Ingall D, Westenfelder G, Yogev R. Peritonsillar abscess complicating infectious mononucleosis. Journal of Pediatrics 1984;104(5):742‐3. - PubMed
Rhen 2005
Rostgaard 2014
Souza 2005
-
- Souza TA, Stollar BD, Sullivan JL, Luzuriaga K, Thorley‐Lawson DA. Peripheral B cells latently infected with Epstein‐Barr virus display molecular hallmarks of classical antigen‐selected memory B cells. Proceedings of the National Academy of Sciences of the United States of America 2005;102(50):18093‐8. [PUBMED: 16330748] - PMC - PubMed
Straus 1993
-
- Straus S. Epstein‐Barr virus infections: biology, pathogenesis and management. Annals of Internal Medicine 1993;118:45. - PubMed
Tattevin 2006
-
- Tattevin P. Increasing incidence of severe Epstein‐Barr virus‐related infectious mononucleosis: surveillance study. Journal of Clinical Microbiology 2006;44(5):1873‐4. [http://jcm.asm.org/content/44/5/1873.long] - PMC - PubMed
Thompson 2005
-
- Thompson SK, Doerr TD, Hengerer AS. Infectious mononucleosis and corticosteroids. Archives of Otolaryngology ‐ Head & Neck Surgery 2005;131:900‐4. - PubMed
Tsikoudas 2006
-
- Tsikoudas A, Davage M, Gardiner Q. Evidence based management of infectious mononucleosis with antibiotics and steroids. The Otorhinolaryngologist 2006;1:29‐31.
Walther 2005
-
- Walther LE, Ilgner J, Oehme A, Schmidt P, Sellhaus B, Gudziol H, et al. Infectious mononucleosis [Die infektiöse Mononukleose]. HNO 2005;53(4):383‐94. - PubMed
White 1995
-
- White PD, Thomas JM, Amess J, Grover SA, Kangro HO, Clare AW. The existence of a fatigue syndrome after glandular fever. Psychological Medicine 1995;25:907‐16. - PubMed
References to other published versions of this review
Candy 2006
Candy 2009
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

