¹H-NMR-Based Metabolomic Study for Identifying Serum Profiles Associated with the Response to Etanercept in Patients with Rheumatoid Arthritis
- PMID: 26558759
- PMCID: PMC4641599
- DOI: 10.1371/journal.pone.0138537
¹H-NMR-Based Metabolomic Study for Identifying Serum Profiles Associated with the Response to Etanercept in Patients with Rheumatoid Arthritis
Abstract
Objective: A considerable proportion of patients with rheumatoid arthritis (RA) do not have a satisfactory response to biological therapies. We investigated the use of metabolomics approach to identify biomarkers able to anticipate the response to biologics in RA patients.
Methods: Due to gender differences in metabolomic profiling, the analysis was restricted to female patients starting etanercept as the first biological treatment and having a minimum of six months' follow-up. Each patient was evaluated by the same rheumatologist before and after six months of treatment. At this time, the clinical response (good, moderate, none) was determined according to the EUropean League Against Rheumatism (EULAR) criteria, based on both erythrocyte sedimentation rate (EULAR-ESR) and C-reactive protein (EULAR-CRP). Sera collected prior and after six months of etanercept were analyzed by 1H-nuclear magnetic resonance (NMR) spectroscopy in combination with multivariate data analysis.
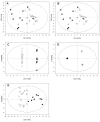
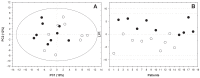
Results: Twenty-seven patients were enrolled: 18 had a good/moderate response and 9 were non responders according to both EULAR-ESR and EULAR-CRP after six months of etanercept. Metabolomic analysis at baseline was able to discriminate good, moderate, and non-responders with a very good predictivity (Q2 = 0.68) and an excellent sensitivity, specificity, and accuracy (100%). In good responders, we found an increase in isoleucine, leucine, valine, alanine, glutamine, tyrosine, and glucose levels and a decrease in 3-hydroxybutyrate levels after six months of treatment with etanercept with respect to baseline.
Conclusion: Our study confirms the potential of metabolomic analysis to predict the response to biological agents. Changes in metabolic profiles during treatment may help elucidate their mechanism of action.
Conflict of interest statement
Figures
Similar articles
-
A Review of Metabolomic Profiling in Rheumatoid Arthritis: Bringing New Insights in Disease Pathogenesis, Treatment and Comorbidities.Metabolites. 2022 Apr 27;12(5):394. doi: 10.3390/metabo12050394. Metabolites. 2022. PMID: 35629898 Free PMC article. Review.
-
Haptoglobin-α1, -α2, vitamin D-binding protein and apolipoprotein C-III as predictors of etanercept drug response in rheumatoid arthritis.Arthritis Res Ther. 2015 Mar 6;17(1):45. doi: 10.1186/s13075-015-0553-1. Arthritis Res Ther. 2015. PMID: 25884688 Free PMC article.
-
Variations in the metabolome in response to disease activity of rheumatoid arthritis.BMC Musculoskelet Disord. 2016 Aug 22;17(1):353. doi: 10.1186/s12891-016-1214-5. BMC Musculoskelet Disord. 2016. PMID: 27549132 Free PMC article.
-
Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register.Rheumatology (Oxford). 2006 Dec;45(12):1558-65. doi: 10.1093/rheumatology/kel149. Epub 2006 May 16. Rheumatology (Oxford). 2006. PMID: 16705046
-
Metabolomics in the development and progression of rheumatoid arthritis: A systematic review.Joint Bone Spine. 2020 Oct;87(5):425-430. doi: 10.1016/j.jbspin.2020.05.005. Epub 2020 May 27. Joint Bone Spine. 2020. PMID: 32473419
Cited by
-
Metabolomic profiles, polygenic risk scores and risk of rheumatoid arthritis: a population-based cohort study in the UK Biobank.RMD Open. 2023 Nov 30;9(4):e003560. doi: 10.1136/rmdopen-2023-003560. RMD Open. 2023. PMID: 38035758 Free PMC article.
-
Relationship Between Inflammation and Metabolism in Patients With Newly Presenting Rheumatoid Arthritis.Front Immunol. 2021 Sep 28;12:676105. doi: 10.3389/fimmu.2021.676105. eCollection 2021. Front Immunol. 2021. PMID: 34650548 Free PMC article.
-
Disease activity and treatment response in early rheumatoid arthritis: an exploratory metabolomic profiling in the NORD-STAR cohort.Arthritis Res Ther. 2025 Jul 26;27(1):156. doi: 10.1186/s13075-025-03616-6. Arthritis Res Ther. 2025. PMID: 40713837 Free PMC article. Clinical Trial.
-
A Review of Metabolomic Profiling in Rheumatoid Arthritis: Bringing New Insights in Disease Pathogenesis, Treatment and Comorbidities.Metabolites. 2022 Apr 27;12(5):394. doi: 10.3390/metabo12050394. Metabolites. 2022. PMID: 35629898 Free PMC article. Review.
-
Metabolomics profiling predicts outcome of tocilizumab in rheumatoid arthritis: an exploratory study.Metabolomics. 2021 Aug 16;17(9):74. doi: 10.1007/s11306-021-01822-2. Metabolomics. 2021. PMID: 34402961 Free PMC article.
References
-
- Egsmose C, Lund B, Borg G, Pettersson H, Berg E, Brodin U, et al. Patients with rheumatoid arthritis benefit from early 2nd line therapy: 5-year followup of a prospective double blind placebo controlled study. J Rheumatol 1995;22:2208–13. - PubMed
-
- van der Heide A, Jacobs JW, Bijlsma JW, Heurkens AH, van Booma-Frankfort C, van der Veen MJ, et al. The effectiveness of early treatment with “second-line” antirheumatic drugs: a randomized, controlled trial. Ann Intern Med 1996;124:699–707. - PubMed
-
- Boers M, Verhoeven AC, Markusse HM, van de Laar MA, Westhovens R, van Denderen JC, et al. Randomised comparison of combined step-down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis. Lancet 1997;350:309–18. - PubMed
-
- Tsakonas E, Fitzgerald AA, Fitzcharles MA, Cividino A, Thorne JC, M’Seffar A, et al. Consequences of delayed therapy with second-line agents in rheumatoid arthritis: a 3 year followup on the hydroxychloroquine in early rheumatoid arthritis (HERA) study. J Rheumatol 2000;27:623–9. - PubMed
-
- Landewe’ RBM, Boers M, Verhoeven AC, Westhovens R, van de Laar MA, Markusse HM, et al. COBRA combination therapy in patients with early rheumatoid arthritis: long-term structural benefits of a brief intervention. Arthritis Rheumatol 2002;46:347–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

