Dopamine/Tyrosine Hydroxylase Neurons of the Hypothalamic Arcuate Nucleus Release GABA, Communicate with Dopaminergic and Other Arcuate Neurons, and Respond to Dynorphin, Met-Enkephalin, and Oxytocin
- PMID: 26558770
- PMCID: PMC4642233
- DOI: 10.1523/JNEUROSCI.0293-15.2015
Dopamine/Tyrosine Hydroxylase Neurons of the Hypothalamic Arcuate Nucleus Release GABA, Communicate with Dopaminergic and Other Arcuate Neurons, and Respond to Dynorphin, Met-Enkephalin, and Oxytocin
Abstract
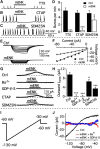
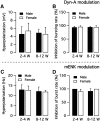
We employ transgenic mice with selective expression of tdTomato or cre recombinase together with optogenetics to investigate whether hypothalamic arcuate (ARC) dopamine/tyrosine hydroxylase (TH) neurons interact with other ARC neurons, how they respond to hypothalamic neuropeptides, and to test whether these cells constitute a single homogeneous population. Immunostaining with dopamine and TH antisera was used to corroborate targeted transgene expression. Using whole-cell recording on a large number of neurons (n = 483), two types of neurons with different electrophysiological properties were identified in the dorsomedial ARC where 94% of TH neurons contained immunoreactive dopamine: bursting and nonbursting neurons. In contrast to rat, the regular oscillations of mouse bursting neurons depend on a mechanism involving both T-type calcium and A-type potassium channel activation, but are independent of gap junction coupling. Optogenetic stimulation using cre recombinase-dependent ChIEF-AAV-DJ expressed in ARC TH neurons evoked postsynaptic GABA currents in the majority of neighboring dopamine and nondopamine neurons, suggesting for the first time substantial synaptic projections from ARC TH cells to other ARC neurons. Numerous met-enkephalin (mENK) and dynorphin-immunoreactive boutons appeared to contact ARC TH neurons. mENK inhibited both types of TH neuron through G-protein coupled inwardly rectifying potassium currents mediated by δ and μ opioid receptors. Dynorphin-A inhibited both bursting and nonbursting TH neurons by activating κ receptors. Oxytocin excited both bursting and nonbursting neurons. These results reveal a complexity of TH neurons that communicate extensively with neurons within the ARC.
Significance statement: Here, we show that the great majority of mouse hypothalamic arcuate nucleus (ARC) neurons that synthesize TH in the dorsomedial ARC also contain immunoreactive dopamine, and show either bursting or nonbursting electrical activity. Unlike rats, the mechanism underlying bursting was not dependent on gap junctions but required T-type calcium and A-type potassium channel activation. Neuropeptides dynorphin and met-enkephalin inhibited dopamine neurons, whereas oxytocin excited them. Most ventrolateral ARC TH cells did not contain dopamine and did not show bursting electrical activity. TH-containing neurons appeared to release synaptic GABA within the ARC onto dopamine neurons and unidentified neurons, suggesting that the cells not only control pituitary hormones but also may modulate nearby neurons.
Keywords: GABA; arcuate nucleus; burst firing; dopamine neuron; neuropeptides; optogenetics.
Copyright © 2015 the authors 0270-6474/15/3514966-17$15.00/0.
Figures











Similar articles
-
Arcuate dopaminergic/GABAergic neurons project within the hypothalamus and to the median eminence.J Neurophysiol. 2024 Sep 1;132(3):943-952. doi: 10.1152/jn.00086.2024. Epub 2024 Aug 7. J Neurophysiol. 2024. PMID: 39108212 Free PMC article.
-
Defining the caudal hypothalamic arcuate nucleus with a focus on anorexic excitatory neurons.J Physiol. 2019 Mar;597(6):1605-1625. doi: 10.1113/JP277152. Epub 2019 Feb 6. J Physiol. 2019. PMID: 30618146 Free PMC article.
-
Hypothalamic arcuate nucleus tyrosine hydroxylase neurons play orexigenic role in energy homeostasis.Nat Neurosci. 2016 Oct;19(10):1341-7. doi: 10.1038/nn.4372. Epub 2016 Aug 22. Nat Neurosci. 2016. PMID: 27548245 Free PMC article.
-
The Hypothalamic Arcuate Nucleus Dopaminergic Neurons: More Than Just Prolactin Secretion.Endocrinology. 2025 Feb 5;166(3):bqaf025. doi: 10.1210/endocr/bqaf025. Endocrinology. 2025. PMID: 39919032 Free PMC article. Review.
-
Oxytocin, GABA, and dopamine interplay in autism.Endocr Regul. 2024 Apr 24;58(1):105-114. doi: 10.2478/enr-2024-0012. Print 2024 Jan 1. Endocr Regul. 2024. PMID: 38656256 Review.
Cited by
-
Dopamine-inhibited POMCDrd2+ neurons in the ARC acutely regulate feeding and body temperature.JCI Insight. 2022 Nov 8;7(21):e162753. doi: 10.1172/jci.insight.162753. JCI Insight. 2022. PMID: 36345942 Free PMC article.
-
A subpopulation of agouti-related peptide neurons exciting corticotropin-releasing hormone axon terminals in median eminence led to hypothalamic-pituitary-adrenal axis activation in response to food restriction.Front Mol Neurosci. 2022 Sep 29;15:990803. doi: 10.3389/fnmol.2022.990803. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36245920 Free PMC article.
-
Central nervous system regulation of organismal energy and glucose homeostasis.Nat Metab. 2021 Jun;3(6):737-750. doi: 10.1038/s42255-021-00408-5. Epub 2021 Jun 21. Nat Metab. 2021. PMID: 34158655 Review.
-
Multiple-scale neuroendocrine signals connect brain and pituitary hormone rhythms.Proc Natl Acad Sci U S A. 2017 Feb 28;114(9):2379-2382. doi: 10.1073/pnas.1616864114. Epub 2017 Feb 13. Proc Natl Acad Sci U S A. 2017. PMID: 28193889 Free PMC article.
-
Arcuate dopaminergic/GABAergic neurons project within the hypothalamus and to the median eminence.J Neurophysiol. 2024 Sep 1;132(3):943-952. doi: 10.1152/jn.00086.2024. Epub 2024 Aug 7. J Neurophysiol. 2024. PMID: 39108212 Free PMC article.
References
-
- Acuna-Goycolea C, Tamamaki N, Yanagawa Y, Obata K, van den Pol AN. Mechanisms of neuropeptide Y, peptide YY, and pancreatic polypeptide inhibition of identified green fluorescent protein-expressing GABA neurons in the hypothalamic neuroendocrine arcuate nucleus. J Neurosci. 2005;25:7406–7419. doi: 10.1523/JNEUROSCI.1008-05.2005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
