Potential Mechanisms Underlying Intercortical Signal Regulation via Cholinergic Neuromodulators
- PMID: 26558772
- PMCID: PMC4642235
- DOI: 10.1523/JNEUROSCI.0629-15.2015
Potential Mechanisms Underlying Intercortical Signal Regulation via Cholinergic Neuromodulators
Abstract
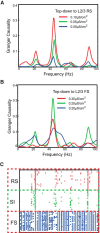
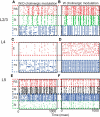
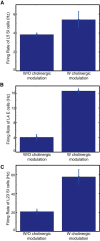
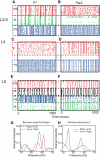
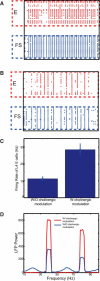
The dynamical behavior of the cortex is extremely complex, with different areas and even different layers of a cortical column displaying different temporal patterns. A major open question is how the signals from different layers and different brain regions are coordinated in a flexible manner to support function. Here, we considered interactions between primary auditory cortex and adjacent association cortex. Using a biophysically based model, we show how top-down signals in the beta and gamma regimes can interact with a bottom-up gamma rhythm to provide regulation of signals between the cortical areas and among layers. The flow of signals depends on cholinergic modulation: with only glutamatergic drive, we show that top-down gamma rhythms may block sensory signals. In the presence of cholinergic drive, top-down beta rhythms can lift this blockade and allow signals to flow reciprocally between primary sensory and parietal cortex.
Significance statement: Flexible coordination of multiple cortical areas is critical for complex cognitive functions, but how this is accomplished is not understood. Using computational models, we studied the interactions between primary auditory cortex (A1) and association cortex (Par2). Our model is capable of replicating interaction patterns observed in vitro and the simulations predict that the coordination between top-down gamma and beta rhythms is central to the gating process regulating bottom-up sensory signaling projected from A1 to Par2 and that cholinergic modulation allows this coordination to occur.
Keywords: cholinergic modulation; computational model; cortical rhythms; dynamical regulation; intercortical communication.
Copyright © 2015 the authors 0270-6474/15/3515000-15$15.00/0.
Figures





References
-
- Bastos AM, Vezoli J, Bosman CA, Schoffelen J-M, Oostenveld R, Dowdall JR, … Fries P. Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron. 2015:390–401. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
