Gonadotrophin-releasing hormone agonist protocols for pituitary suppression in assisted reproduction
- PMID: 26558801
- PMCID: PMC10759000
- DOI: 10.1002/14651858.CD006919.pub4
Gonadotrophin-releasing hormone agonist protocols for pituitary suppression in assisted reproduction
Update in
-
Gonadotropin-releasing hormone agonist protocols for pituitary suppression in assisted reproduction.Cochrane Database Syst Rev. 2025 Jan 9;1(1):CD006919. doi: 10.1002/14651858.CD006919.pub5. Cochrane Database Syst Rev. 2025. PMID: 39783453
Abstract
Background: Gonadotrophin-releasing hormone agonists (GnRHa) are commonly used in assisted reproduction technology (ART) cycles to prevent a luteinising hormone surge during controlled ovarian hyperstimulation (COH) prior to planned oocyte retrieval, thus optimising the chances of live birth.
Objectives: To evaluate the effectiveness of the different GnRHa protocols as adjuncts to COH in women undergoing ART cycles.
Search methods: We searched the following databases from inception to April 2015: the Cochrane Menstrual Disorders and Subfertility Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (2015, Issue 3), MEDLINE, EMBASE, CINAHL, PsycINFO, and registries of ongoing trials. Reference lists of relevant articles were also searched.
Selection criteria: We included randomised controlled trials (RCTs) comparing any two protocols of GnRHa used in in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) cycles in subfertile women.
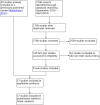
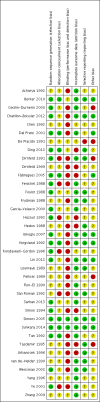
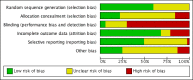
Data collection and analysis: Two review authors independently selected studies, assessed trial eligibility and risk of bias, and extracted the data. The primary outcome measure was number of live births or ongoing pregnancies per woman/couple randomised. Secondary outcome measures were number of clinical pregnancies, number of oocytes retrieved, dose of gonadotrophins used, adverse effects (pregnancy losses, ovarian hyperstimulation, cycle cancellation, and premature luteinising hormone (LH) surges), and cost and acceptability of the regimens. We combined data to calculate odds ratios (OR) for dichotomous variables and mean differences (MD) for continuous variables, with 95% confidence intervals (CIs). We assessed statistical heterogeneity using the I² statistic. We assessed the overall quality of the evidence for the main comparisons using 'Grading of Recommendations Assessment, Development and Evaluation' (GRADE) methods.
Main results: We included 37 RCTs (3872 women), one ongoing trial, and one trial awaiting classification. These trials made nine different comparisons between protocols. Twenty of the RCTs compared long protocols and short protocols. Only 19/37 RCTs reported live birth or ongoing pregnancy.There was no conclusive evidence of a difference between a long protocol and a short protocol in live birth and ongoing pregnancy rates (OR 1.30, 95% CI 0.94 to 1.81; 12 RCTs, n = 976 women, I² = 15%, low quality evidence). Our findings suggest that in a population in which 14% of women achieve live birth or ongoing pregnancy using a short protocol, between 13% and 23% will achieve live birth or ongoing pregnancy using a long protocol. There was evidence of an increase in clinical pregnancy rates (OR 1.50, 95% CI 1.18 to 1.92; 20 RCTs, n = 1643 women, I² = 27%, moderate quality evidence) associated with the use of a long protocol.There was no evidence of a difference between the groups in terms of live birth and ongoing pregnancy rates when the following GnRHa protocols were compared: long versus ultrashort protocol (OR 1.78, 95% CI 0.72 to 4.36; one RCT, n = 150 women, low quality evidence), long luteal versus long follicular phase protocol (OR 1.89, 95% CI 0.87 to 4.10; one RCT, n = 223 women, low quality evidence), when GnRHa was stopped versus when it was continued (OR 0.75, 95% CI 0.42 to 1.33; three RCTs, n = 290 women, I² = 0%, low quality evidence), when the dose of GnRHa was reduced versus when the same dose was continued (OR 1.02, 95% CI 0.68 to 1.52; four RCTs, n = 407 women, I² = 0%, low quality evidence), when GnRHa was discontinued versus continued after human chorionic gonadotrophin (HCG) administration in the long protocol (OR 0.89, 95% CI 0.49 to 1.64; one RCT, n = 181 women, low quality evidence), and when administration of GnRHa lasted for two versus three weeks before stimulation (OR 1.14, 95% CI 0.49 to 2.68; one RCT, n = 85 women, low quality evidence). Our primary outcomes were not reported for any other comparisons.Regarding adverse events, there were insufficient data to enable us to reach any conclusions except about the cycle cancellation rate. There was no conclusive evidence of a difference in cycle cancellation rate (OR 0.95, 95% CI 0.59 to 1.55; 11 RCTs, n = 1026 women, I² = 42%, low quality evidence) when a long protocol was compared with a short protocol. This suggests that in a population in which 9% of women would have their cycles cancelled using a short protocol, between 5.5% and 14% will have cancelled cycles when using a long protocol.The quality of the evidence ranged from moderate to low. The main limitations in the evidence were failure to report live birth or ongoing pregnancy, poor reporting of methods in the primary studies, and imprecise findings due to lack of data. Only 10 of the 37 included studies were conducted within the last 10 years.
Authors' conclusions: When long GnRHa protocols and short GnRHa protocols were compared, we found no conclusive evidence of a difference in live birth and ongoing pregnancy rates, but there was moderate quality evidence of higher clinical pregnancy rates in the long protocol group. None of the other analyses showed any evidence of a difference in birth or pregnancy outcomes between the protocols compared. There was insufficient evidence to make any conclusions regarding adverse effects.
Conflict of interest statement
Charalampos S Siristatidis: nothing to declare. Ahmed Gibreel: nothing to declare. George Basios: nothing to declare. Abha Maheshwari: nothing to declare. Siladitya Bhattacharya: nothing to declare.
Figures













































Update of
-
Gonadotrophin-releasing hormone agonist protocols for pituitary suppression in assisted reproduction.Cochrane Database Syst Rev. 2011 Aug 10;(8):CD006919. doi: 10.1002/14651858.CD006919.pub3. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2015 Nov 09;(11):CD006919. doi: 10.1002/14651858.CD006919.pub4. PMID: 21833958 Updated.
References
References to studies included in this review
Acharya 1992 {published data only}
-
- Acharya U, Small J, Randall J, Hamilton M, Templeton A. Prospective study of short and long regimens of gonadotropin‐releasing hormone agonist in in vitro fertilization program. Fertility and Sterility 1992;57(4):815‐8. [PUBMED: 555693] - PubMed
Berker 2010 {published data only}
-
- Berker B, Duvan Cİ, Kaya C, Aytaç R, Satıroğlu H. Comparison of the ultrashort gonadotropin‐releasing hormone agonist‐antagonist protocol with microdose flare‐up protocol in poor responders: a preliminary study. Journal of the Turkish German Gynecological Association 2010;11(4):187‐93. [PUBMED: 24591934] - PMC - PubMed
Cedrin‐Durnerin 2000 {published data only}
-
- Cedrin‐Durnerin I, Bidart JM, Robert P, Wolf JP, Uzan M, Hugues JN. Consequences on gonadotrophin secretion of an early discontinuation of gonadotrophin‐releasing hormone agonist administration in short term protocol for in vitro fertilization. Human Reproduction (Oxford, England) 2000;15(5):1009‐14. [PUBMED: 10783343] - PubMed
Chatillon‐Boissier 2012 {published data only}
-
- Chatillon‐Boissier K, Genod A, Denis‐Belicard E, Felloni B, Chene G, Seffert P, et al. Prospective randomised study of long versus short agonist protocol with poor responder patients during in vitro fertilization [Étude prospective randomisée protocoles agonistes long versus court chez les patientes mauvaises répondeuses en fécondation in vitro]. Gynécologie Obstétrique & Fertilité 2012;40(11):652‐57. [PUBMED: 22342506] - PubMed
Chen 1992 {published data only}
-
- Chen SU, Yang YS, Ho HN, Hwang JL, Lien YR, Lin HR, et al. Comparison of long‐term versus three‐day leuprolide acetate for ovarian stimulation in human in vitro fertilization program. Journal of Reproduction and Fertility 1992;1:9‐16.
Dal Prato 2001 {published data only}
-
- Dal Prato L, Borini A, Trevisi MR, Bonu MA, Sereni E, Flamigni C. Effect of reduced dose of triptorelin at the start of ovarian stimulation on the outcome of IVF: a randomized study. Human Reproduction (Oxford, England) 2001;16(7):1409‐14. [PUBMED: 11425821] - PubMed
De Placido 1991 {published data only}
-
- Placido G, Zullo F, Colacurcu N, Perrone D, Carravetta C, Montemagno U. Long acting versus daily administration GnRH analogs in IVF (abstract). Human Reproduction. 7th Annual Meeting of the ESHRE and 7th World Congress on IVF and Assisted Procreation 1991;6(Suppl 1):339‐40 (abs # p482).
Ding 2013 {published data only}
-
- Ding LJ, Wang B, Shen XY, Yan GJ, Zhang NY, Hu YL, Sun HX. Withdrawal of GnRH agonist decreases oestradiol and VEGF concentrations in high responders. Reproductive BioMedicine Online 2013;27(2):131‐9. [PUBMED: 23764202] - PubMed
Dirnfeld 1991 {published data only}
-
- Dirnfeld M, Gonen Y, Lissak A, Goldman S, Koifman M, Sorokin Y, Abramovici H. A randomized prospective study on the effect of short and long buserelin treatment in women with repeated unsuccessful in vitro fertilization (IVF) cycles due to inadequate ovarian response. Journal of In Vitro Fertilization and Embryo Transfer: IVF 1991;8(6):339‐43. [PUBMED: 1770275] - PubMed
Dirnfeld 1999 {published data only}
-
- Dirnfeld M, Fruchter O, Yshai D, Lissak A, Ahdut A, Abramovici H. Cessation of gonadotropin‐releasing hormone analogue (GnRH‐a) upon down‐regulation versus conventional long GnRH‐a protocol in poor responders undergoing in vitro fertilization. Fertility and Sterility 1999;72(3):406‐11. [PUBMED: 10519608] - PubMed
Fábregues 2005 {published data only}
-
- Fábregues F, Peñarrubia J, Creus M, Casamitjana R, Vanrell JA, Balasch J. Effect of halving the daily dose of triptorelin at the start of ovarian stimulation on hormone serum levels and the outcome of in vitro fertilization. Fertility and Sterility 2005;83(3):785‐8. [PUBMED: 15749520] - PubMed
Fenichel 1988 {published data only}
-
- Fenichel P, Grimaldi M, Hieronimus S, Olivero JF, Donzeau A, Benoit B, et al. Systematic inhibition of the luteinizing hormone with a gonadoliberin analog, triptorelin, during ovarian stimulation for fertilization in vitro. Choice of protocol [Inhibition systematique de l`hormone luteinisante par un analogue de la gonadoliberine, la triptoreline, au cours de la stimulation ovarienne pour fecondation in vitro: choix du protocole]. Presse Médicale 1988;17(15):719‐22. [PUBMED: 2968548] - PubMed
Foulot 1988 {published data only}
-
- Foulot H, Dubisson JB, Ranoux C, Aubriot FX, Poirot C. Randomized study comparing the short and long protocols of buserelin in 100 in vitro fertilization cycles [Etude randomisee entre protocole court et protocole long de buserelin concernant 100 cycles de fecondation in vitro]. Contraception, Fertilite, Sexualite 1988;16:628‐9.
Frydman 1988 {published data only}
-
- Frydman R, Belaisch‐Allart J, Parneix I, Forman R, Hazout A, Testart J. Comparison between flare up and down regulation effects of luteinizing hormone‐releasing hormone agonists in an in vitro fertilization program. Fertility and Sterility 1988;50(3):471‐5. [PUBMED: 2970407] - PubMed
Garcia‐Velasco 2000 {published data only}
-
- Garcia‐Velasco JA, Isaza V, Requena A, Martinez‐Salazar FJ, Landazábal A, Remohi J, et al. High doses of gonadotrophins combined with stop versus non‐stop protocol of GnRH analogue administration in low responder IVF patients: a prospective, randomized controlled trial. Human Reproduction (Oxford, England) 2000;15(11):2292‐6. [PUBMED: 11056121] - PubMed
Hazout 1993 {published data only}
-
- Hazout A, Ziegler D, Cornel C, Fernandez H, Lelaidier C, Frydman R. Comparison of short 7 day and prolonged treatment with gonadotropin‐releasing hormone agonist desensitization for controlled ovarian hyperstimulation. Ferility and Sterility 1993;59(3):596‐600. [PUBMED: 8458463] - PubMed
Hedon 1988 {published data only}
-
- Hedon B, Arnal F, Badoc E, Boulot P, Huet JM, Fries N, et al. Randomized comparison of the long and short protocols for ovarian stimulation in association with GnRH agonist for in vitro fertilization [Comparison randomisee protocole long‐protocole court dans les stimulations de l`ovaire en association avec un agoniste de la GnRH en vue de fecondation in vitro]. Contraception, Fertilite, Sexualite 1988;16:624‐7.
Isikoglu 2007 {published data only}
-
- Isikoglu M, Ozgur K, Oehninger S. Extension of GnRH agonist through the luteal phase to improve the outcome of intracytoplasmic sperm injection. The Journal of Reproductive Medicine 2007;52(7):639‐44. [PUBMED: 17847764] - PubMed
Kingsland 1992 {published data only}
-
- Kingsland C, Tan SL, Bickerton N, Mason B, Campbell S. The routine use of gonadotropin‐releasing hormone agonists for all patients undergoing in vitro fertilization. Is there any medical advantage? A prospective randomized study. Fertility and Sterility 1992;57(4):804‐9. [PUBMED: 1555691] - PubMed
Kondaveeti‐Gordon 1996 {published data only}
-
- Kondaveeti‐Gordon U, Harrison RF, Barry‐Kinsella C, Gordon AC, Drudy L, Cottell E. A randomized prospective study of early follicular or midluteal initiation of long protocol gonadotropin‐releasing hormone in an in vitro fertilization program. Fertility and Sterility 1996;66(4):582‐6. [PUBMED: 8816620] - PubMed
Lin 2013 {published data only}
-
- Lin H, Li Y, Li L, Zhang Q, Wang W, Chen X, et al. Effect of delayed initiation of gonadotropin in luteal long protocol on in vitro fertilization. Gynecological Endocrinology: the Official Journal of the International Society of Gynecological Endocrinology 2013;29(9):846‐50. [PUBMED: 23865696] - PubMed
Loumaye 1989 {published data only}
-
- Loumaye E, Vankrieken L, Depreester S, Psalti I, Cooman S, Thomas K. Hormonal changes induced by short‐term administration of gonadotropin‐releasing hormone agonist during ovarian hyperstimulation for in vitro fertilization and their consequences for embryo development. Fertility and Sterility 1989;51(1):105‐11. [PUBMED: 2491990] - PubMed
Pellicer 1989 {published data only}
-
- Pellicer A, Simón C, Miró F, Castellvi RM, Ruiz A, Ruiz M, et al. Ovarian response and outcome of in‐vitro fertilization in patients treated with gonadotrophin‐releasing hormone analogues in different phases of menstrual cycle. Human Reproduction (Oxford, England) 1989;4(3):285‐9. [PUBMED: 2497135] - PubMed
Ron‐El 1990 {published data only}
-
- Ron‐El R, Herman A, Golan A, Ven H, Caspi E, Diedrich K. The comparison of early follicular and midluteal administration of long acting gonadotropin‐releasing hormone agonist. Fertility and Sterility 1990;54(2):233‐7. [PUBMED: 2143145] - PubMed
San Roman 1992 {published data only}
-
- San Roman GA, Surrey ES, Judd HL, Kerin JF. A prospective randomized comparison of luteal phase versus concurrent follicular phase initiation of gonadotropin‐releasing hormone agonist for in‐vitro fertilization. Fertility and Sterility 1992;58(4):744‐9. [PUBMED: 1426320] - PubMed
Sarhan 2013 {published data only}
-
- Sarhan AM, Harira MM, Alshazly SM. Comparing stimulation requirements and final outcome between early follicular and mid luteal‐starting of pituitary suppression in the long gonadotropin releasing hormone agonist protocol. Fertility and Sterility 2013;100 Suppl (2):S472. - PubMed
Simon 1994 {published data only}
-
- Simon A, Benshushan A, Shushan A, Zajicek G, Dorembus D, Lewin A, et al. A comparison between a standard and reduced dose of D‐Trp‐6‐luteinizing hormone‐releasing hormone administered after pituitary suppression for in‐vitro fertilization. Human Reproduction (Oxford, England) 1994;9(10):1813‐7. [PUBMED: 7844208] - PubMed
Simons 2005 {published data only}
-
- Simons AH, Roelofs HJ, Schmoutziguer AP, Roozenburg BJ, van't Hof‐van den Brink EP, Schoonderwoerd SA. Early cessation of triptorelin in in vitro fertilization: a double blind, randomized study. Fertility and Sterility 2005;83(4):889‐96. [PUBMED: 15820796] - PubMed
Sunkara 2014 {published data only}
-
- Sunkara SK, Coomarasamy A, Faris R, Braude P, Khalaf Y. Long gonadotropin‐releasing hormone agonist versus short agonist versus antagonist regimens in poor responders undergoing in vitro fertilization: a randomized controlled trial. Fertility and Sterility 2014;101(1):147‐53. [PUBMED: 24188873] - PubMed
-
- Sunkara SK, Coomarasamy A, Khalaf Y, Braude P. A three‐arm randomised controlled trial comparing Gonadotrophin Releasing Hormone (GnRH) agonist long regimen versus GnRH agonist short regimen versus GnRH antagonist regimen in women with a history of poor ovarian response undergoing in vitro fertilisation (IVF) treatment: Poor responders intervention trial (PRINT). Reproductive Health 2007; Vol. 4, issue 12. [PUBMED: 18163902] - PMC - PubMed
-
- Sunkara SK, Coomarasamy AA, Faris RA, Braude PA, Khalaf Y. Effectiveness of the GnRH agonist long, GnRH agonist short and GnRH antagonist regimens in poor responders undergoing IVF treatment: a three arm randomised controlled trial. Human Reproduction 2013;28:311‐356.
Tan 1992 {published data only}
-
- Tan SL, Kingsland C, Campbell S, Mills C, Bradfield J, Alexander N, et al. The long protocol of administration of gonadotropin‐releasing hormone agonist is superior to the short protocol for ovarian stimulation for in‐vitro fertilization. Fertility and Sterility 1992;57(4):810‐4. [PUBMED: 1555692] - PubMed
Tasdemir 1995 {published data only}
-
- Tasdemir M, Tasdemir I, Kodama H, Higuchi M. Is long‐protocol gonadotropin releasing hormone agonist administration superior to the short protocol in ovarian stimulation for in‐vitro fertilization?. International Journal of Fertility and Menopausal Studies 1995;40(1):25‐8. [PUBMED: 7749431] - PubMed
Urbancsek 1996 {published data only}
-
- Urbancsek J, Witthaus E. Midluteal buserelin is superior to early follicular phase buserelin in combined gonadotropin‐releasing hormone analog and gonadotropin stimulation in in vitro fertilization. Fertility and Sterility 1996;65(5):966‐71. [PUBMED: 8612858] - PubMed
van de‐Helder 1990 {published data only}
-
- de‐Helder AB, Helmerhorst FM, Blankhart A, Brand R, Waegemaekers C, Naaktgeboren N. Comparison of ovarian stimulation regimens for in vitro fertilization (IVE) with and without a gonadotropin‐releasing hormone (GnRH) agonist: results of a randomized study. Journal of In Vitro Fertilization and Embryo Transfer: IVF 1990;7(6):358‐62. [PUBMED: 2127604] - PubMed
Weissman 2003 {published data only}
-
- Weissman A, Farhi J, Royburt M, Nahum H, Glezerman M, Levran D. Prospective evaluation of two stimulation protocols for low responders who were undergoing in vitro fertilization‐embryo transfer. Fertility and Sterility 2003;79(4):886‐92. [PUBMED: 12749425] - PubMed
Yang 1996 {published data only}
-
- Yang TS, Tsan SH, Wang BC, Chang SP, Ng HT. The evaluation of a new 7‐day gonadotropin‐relasing hormone agonist protocol in the controlled ovarian hyperstimulation for in vitro fertilization. The Journal of Obstetrics and Gynaecology Research 1996;22(2):133‐7. [PUBMED: 8697342] - PubMed
Ye 2001 {published data only}
-
- Ye H, Huang G, Pei L. A prospective, randomized controlled study comparing the effects of gonadotropin‐releasing hormone agonist long and short protocols for in vitro fertilization. Zhonghua Fu Chan Ke Za Zhi 2001;36(4):222‐5. [PUBMED: 11783366] - PubMed
Zhang 2009 {published data only}
-
- Zhang J, Li Y, Liu J, Liu De, Liu N, Chen X. Effect of 7‐day gonadotropin‐releasing hormone agonist protocol on IGF‐II and IGFBP‐4 levels in the follicular fluid. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2009;34(3):190‐4. [PUBMED: 19349671] - PubMed
References to studies excluded from this review
Abd Rabo 2012 {published data only}
-
- Abd Rabbo MH, Elmaghraby HA, Mashali NA, Abdel Moneim ME. Effect of aromatase inhibitor (letrozole) with long agonist protocol on the results of ICSI/ET in females with minimal and mild endometriosis. Alexandria Journal of Medicine 2012;48(4):303‐7. [DOI: 10.1016/j.ajme.2012.04.001] - DOI
Aflatoonian 2012 {published data only}
-
- Aflatoonian A, Yousefnejad F, Eftekhar M, Mohammadian F. Efficacy of low‐dose hCG in late follicular phase in controlled ovarian stimulation usingGnRH agonist protocol. Archives of Gynecology and Obstetrics 2012;286(3):771‐5. [PUBMED: 22619027] - PubMed
Albuquerque 2013 {published data only}
-
- Albuquerque LE, Tso LO, Saconato H, Albuquerque MC, Macedo CR. Depot versus daily administration of gonadotrophin‐releasing hormone agonist protocols for pituitary down regulation in assisted reproduction cycles. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD002808.pub3] - DOI - PMC - PubMed
Antoine 1990 {published data only}
-
- Antoine JM, Firmin C, Alvarez S, Cornet D, Tibi C, Mandelbaum J, et al. Ovarian stimulation for in vitro fertilization using LHRH agonists: comparison of plasma and intra‐follicular hormone profiles using "short" and "long" protocols [Stimulation ovarienne pour fecondation in vitro utilisant les agonistes de la LHRH: Comparison des profils hormonaux plasmatiques et intra‐folliculaires des protocoles courts et longs]. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1990;19(8):1035‐40. [PUBMED: 2150525] - PubMed
Azem 2010 {published data only}
-
- Azem F, Bloch M, Kuvalsky D, Wagman I, Bibi G, Amit A. Comparison of short and long GnRH agonist protocols using recombinant FSH for IVF/ICSI: a controlled prospective study. Fertility and Sterility 2010;94(4 Suppl):S163.
Beckers 2000 {published data only}
-
- Beckers NG, Laven JS, Eijkemans MJ, Fauser BC. Follicular and luteal phase characteristics following early cessation of gonadotrophin‐releasing hormone agonist during ovarian stimulation for in‐vitro fertilization. Human Reproduction (Oxford, England) 2000;15(1):43‐9. [PUBMED: 10611186] - PubMed
Bloch 2011 {published data only}
-
- Bloch M, Azem F, Aharonov I, Ben Avi I, Yagil Y, Schreiber S, et al. GnRH‐agonist induced depressive and anxiety symptoms during in vitro fertilization‐embryo transfer cycles. Fertility and Sterility 2011;95(1):307‐9. [PUBMED: 20801439] - PubMed
Braendle 1989 {published data only}
-
- Braendle W, Lindner C, Lichtenberg V, Goepel E, Bettendorf G. Endocrine profiles and luteal function during GnRH‐analogue/HMG therapy. Human Reproduction (Oxford, England) 1989;4(8 Suppl):121‐6. [PUBMED: 2533217] - PubMed
Buvat 1993 {published data only}
-
- Buvat J, Marcolin G, Guittard C, Herbaut M, Louvet A, Renouard O. Requiem for the short protocol? [Requiem pour le protocol]. Contraception, Fertilite, Sexualite 1993;21:436.
Cambiaghi 2011 {published data only}
-
- Cambiaghi AS, Leao RBF, Castellotti DS, Nascimento P. Triptorelin every other day can prevent premature LH surge: a strategy to reduce the daily gonadotrophin‐releasing hormone agonists injections. Fertility and Sterility 2011;96(3 Suppl):S175.
Check 1992 {published data only}
-
- Check JH, Nowroozi K, Chase JS. Comparison of short versus long‐term leuprolide acetate‐human menopausal gonadotrophin hyperstimulation in in‐vitro fertilization patients. Human Reproduction (Oxford, England) 1992;7(1):31‐4. [PUBMED: 1551954] - PubMed
Cheon 2008 {published data only}
-
- Cheon KW, Song SJ, Choi BC, Lee SC, Lee HB, Yu SY, et al. Comparison of clinical efficacy between a single administration of long‐acting gonadotrophin‐releasing hormone agonist (GnRHa) and daily administrations of short‐acting GnRHa in in vitro fertilization‐embryo transfer cycles. Journal of Korean Medical Science 2008;23(4):662‐6. [PUBMED: 18756054] - PMC - PubMed
Corson 1992 {published data only}
-
- Corson SL, Batzer FR, Gocial B, Eisenberg E, Huppert LC, Nelson JR. Leuprolide acetate‐prepared in vitro fertilization‐gamete intrafallopian transfer cycles: efficacy versus controls and cost analysis. Fertility and Sterility 1992;57(3):601‐5. [PUBMED: 1740205] - PubMed
Dessolle 2011 {published data only}
-
- Dessolle L, Ferrier D, Colombel A, Fréour T, Jean M, Barrière P. Prolonging GnRH‐agonist to achieve ovarian suppression does not compromise the results of a long protocol. European Journal of Obstetrics, Gynecology and Reproductive Biology 2011;159(1):111‐4. [PUBMED: 21763059] - PubMed
Devroey 1994 {published data only}
-
- Devroey P, Mannaerts B, Smitz J, Coelingh Bennink H, Steirteghem A. Clinical outcome of a pilot efficacy study on recombinant human follicle‐stimulating hormone (Org 32489) combined with various gonadotrophin‐releasing hormone agonist regimens. Human Reproduction (Oxford, England) 1994;9(6):1064‐9. [PUBMED: 7962377] - PubMed
Dor 1992 {published data only}
-
- Dor J, Shulman A, Pariente C, Levran D, Bider D, Menashe Y, et al. The effect of gonadotropin‐releasing hormone agonist on the ovarian response and in vitro fertilization results in polycystic ovarian syndrome: a prospective study. Fertility and Sterility 1992;57(2):366‐71. [PUBMED: 1531200] - PubMed
Eftekhar 2013 {published data only}
Elgendy 1998 {published data only}
-
- Elgendy M, Afnan M, Holder R, Lashen H, Afifi Y, Lenton W, et al. Reducing the dose of gonadotrophin‐releasing hormone agonist on starting ovarian stimulation: effect on ovarian response and in‐vitro fertilization outcome. Human Reproduction (Oxford, England) 1998;13(9):2382‐5. [PUBMED: 9806253] - PubMed
Faber 1998 {published data only}
-
- Faber BM, Mayer J, Cox B, Jones D, Toner JP, Oehninger S, Muasher SJ. Cessation of gonadotropin‐releasing hormone agonist therapy combined with high‐dose gonadotropin stimulation yields favourable pregnancy results in low responders. Fertility and Sterility 1998;69(5):826‐30. [PUBMED: 9591487] - PubMed
Ferraretti 1996 {published data only}
-
- Ferraretti AP, Magli C, Feliciani E, Montanaro N, Gianaroli L. Relationship of timing of agonist administration in the cycle phase to the ovarian response to gonadotropins in the long down‐regulation protocols for assisted reproductive technologies. Fertility and Sterility 1996;65(1):114‐21. [PUBMED: 8557125] - PubMed
Fujii 1997 {published data only}
-
- Fujii S, Sagara M, Kudo H, Kagiya A, Sato S, Saito Y. A prospective randomized comparison between long and discontinuous‐long protocols of gonadotropin‐releasing hormone agonist for in vitro fertilization. Fertility and Sterility 1997;67(6):1166‐8. [PUBMED: 9176463] - PubMed
Garcia 1990 {published data only}
-
- Garcia JE, Padilla SL, Bayati J, Baramki TA. Follicular phase gonadotropin‐releasing hormone agonist and human gonadotropins: a better alternative for ovulation induction in in vitro fertilization. Fertility and Sterility 1990;53(2):302‐5. [PUBMED: 2105247] - PubMed
Gersak 1994 {published data only}
-
- Gersak K, Meden‐Vrtovec H, Tomazevic T. The effects of gonadotrophin‐releasing hormone agonist on follicular development in patients with polycystic ovary syndrome in an in‐vitro fertilization and embryo transfer programme. Human Reproduction (Oxford, England) 1994;9(9):1596‐9. [PUBMED: 7836506] - PubMed
Gianaroli 1994 {published data only}
-
- Gianaroli L, Ferraretti AP, Feliciani E, Tabanelli C, Magli C, Fortini D. Prospective randomized study of D‐Trp6‐ LHRH versus buserelin in long desensitization protocols for medically assisted conception cycles. Human Reproduction (Oxford, England) 1994;9(2):220‐5. [PUBMED: 8027275] - PubMed
Gizzo 2014 {published data only}
-
- Gizzo S, Andrisani A, Esposito F, Noventa M, Gangi S, Angioni S, et al. Which luteal phase support is better for each IVF stimulation protocol to achieve the highest pregnancy rate? A superiority randomized clinical trial. Gynecological Endocrinology: the official journal of the International Society of Gynecological Endocrinology 2014;30:1‐7. [PUBMED: 25268567] - PubMed
Harrison 1994 {published data only}
-
- Harrison RF, Kondaveeti U, Barry‐Kinsella C, Gordon A, Drudy L, Cottell E, et al. Should gonadotropin‐releasing hormone down‐regulation therapy be routine in in vitro fertilization?. Fertility and Sterility 1994;62(3):568‐73. [PUBMED: 8062954] - PubMed
Huang 2012 {published data only}
-
- Huang R, Fang C, Xu S, Yi Y, Liang X. Premature progesterone rise negatively correlated with live birth rate in IVF cycles with GnRH agonist: an analysis of 2,566 cycles. Fertility and Sterility 2012;98(3):664‐70.e2. [PUBMED: 22704632] - PubMed
Jinno 1996 {published data only}
-
- Jinno M, Yoshimura Y, Ubukata Y, Nakamura Y. A novel method of ovarian stimulation for in vitro fertilization: bromocriptine‐rebound method. Fertility and Sterility 1996;66(2):271‐4. [PUBMED: 8690115] - PubMed
Jinno 2009 {published data only}
-
- Jinno M, Watanabe A, Hirohama J, Eguchi N, Hatakeyama N. Diminished but significant mid‐cycle LH surge can be induced in the long protocol of GNRH agonist and HMG regimen, resulting in a great increase in the rate of ongoing pregnancy. Fertility and Sterility 2009;92(3 Suppl):S235.
Ku 2005 {published data only}
-
- Ku SY, Choi YS, Jee BC, Suh CS, Choi YM, Kim JG, et al. A preliminary study on reduced dose (33 or 25 g) gonadotropin‐releasing hormone agonist long protocol for multi follicular ovaria stimulation in patients with high basal serum follicular‐stimulating hormone levels undergoing in vitro fertilization‐embryo transfer. Gynaecological Endocrinology: the official journal of the International Society of Gynecological Endocrinology 2005;21:227‐31. - PubMed
Kubik 1990 {published data only}
-
- Kubik CJ, Guzick DS, Berga SL, Zeleznik AJ. Randomized, prospective trial of leuprolide acetate and conventional superovulation in first cycle of in vitro fertilization and gamete intrafallopian transfer. Fertility and Sterility 1990;54(5):836‐41. [PUBMED: 2121551] - PubMed
Kuc 2011 {published data only}
-
- Kuć P, Kuczyńska A, Topczewska M, Tadejko P, Kuczyński W. The dynamics of endometrial growth and the triple layer appearance in three different controlled ovarian hyperstimulation protocols and their influence on IVF outcomes. Gynecological Endocrinology: the official journal of the International Society of Gynecological Endocrinology 2011;27(11):867‐73. [PUBMED: 21231852] - PubMed
Li 2012 {published data only}
Liu 2012 {published data only}
-
- Liu N, Ma Y, Li R, Jin H, Li M, Huang X, Feng HL, Qiao J. Comparison of follicular fluid amphiregulin and EGF concentrations in patients undergoing IVF with different stimulation protocols. Endocrine 2012;42(3):708‐16. [PUBMED: 22678853] - PubMed
Lorusso 2004 {published data only}
-
- Lorusso F, Depalo R, Selvaggi L. Relationship between gonadotropin releasing hormone agonist dosage and in vitro fertilization outcome. Gynecological Endocrinology: the official Journal of the International Society of Gynecological Endocrinology 2004;18(2):69‐73. [PUBMED: 15195497] - PubMed
Loutradis 1998 {published data only}
-
- Loutradis D, Drakakis P, Kallianidis K, Bletsa R, Milingos S, Makris N, et al. The effect of the duration of GnRH‐agonist down regulation before ovarian stimulation on the biological and clinical outcome after intracytoplasmic sperm injection. European Journal of Obstetrics Gynecology and Reproductive Biology 1998;80(2):251‐5. [PUBMED: 9846679] - PubMed
Marcus 1993 {published data only}
-
- Marcus SF, Brinsden PR, Macnamee M, Rainsbury PA, Elder KT, Edwards RG. Comparative trial between an ultra‐short and long protocol of luteinizing hormone‐releasing hormone agonist for ovarian stimulation in in‐vitro fertilization. Human Reproduction (Oxford, England) 1993;8(2):238‐43. [PUBMED: 8473427] - PubMed
Maroulis 1991 {published data only}
-
- Maroulis GB, Emery M, Verkauf BS, Saphier A, Bernhisel M, Yeko TR. Prospective randomized study of human menotropin versus a follicular and a luteal phase gonadotropin‐releasing hormone analog‐human menotropin stimulation protocols for in vitro fertilization. Fertility and Sterility 1991;55(6):1157‐64. [PUBMED: 1903732] - PubMed
McKenna 1989 {published data only}
-
- McKenna KM, Foster P, McBain J, Martin M, Johnston WI. Combined treatment with gonadotrophin releasing hormone agonist and gonadotrophins in poor responders to hyperstimulation for in vitro fertilization (IVF): clinical and endocrine results. Australian and New Zealand Journal of Obstetrics and Gynaecology 1989;29(4):428‐32. [PUBMED: 2517193] - PubMed
Mochtar 2011 {published data only}
-
- Mochtar MH, Custers IM, Koks CA, Bernardus RE, Verhoeve HR, Mol BW, et al. Timing oocyte collection in GnRH agonists down‐regulated IVF and ICSI cycles: a randomized clinical trial. Human Reproduction (Oxford, England) 2011;26(5):1091‐6. [PUBMED: 21362684] - PubMed
NCT00436319 {published data only}
-
- NCT00436319. Effect of the duration of GnRH‐analogue downregulation on pregnancy rates in IVF. clinicaltrials.gov/ct2/show/NCT00436319 (accessed 2015).
NCT02342197 {published data only}
-
- NCT02342197. Comparative study between minidose long protocol and microdose flare protocol in controlled ovarian hyperstimulation for poor responders in ICSI cycles. clinicaltrials.gov/ct2/show/NCT02342197 (accessed 04/2015).
Neuspiller 1998 {published data only}
-
- Neuspiller F, Levy M, Remohí J, Ruiz A, Simón C, Pellicer A. The use of long‐ and short‐acting forms of gonadotrophin‐releasing hormone analogues in women undergoing oocyte donation. Human Reproduction (Oxford, England) 1998;13(5):1148‐51. [PUBMED: 9647536] - PubMed
Norman 1991 {published data only}
-
- Norman RJ, Warnes GM, Wang X, Kirby CA, Matthews CD. Differential effects of gonadotrophin‐releasing hormone agonists administered as desensitizing or flare protocols on hormonal function in the luteal phase of hyperstimulated cycles. Human Reproduction (Oxford, England) 1991;6(2):206‐13. [PUBMED: 1905309] - PubMed
Padilla 1991 {published data only}
-
- Padilla SL, Smith RD, Garcia JE. The Lupron screening test: tailoring the use of leuprolide acetate in ovarian stimulation for in vitro fertilization. Fertility and Sterility 1991;56(1):79‐83. [PUBMED: 1906020] - PubMed
Pantos 1994 {published data only}
-
- Pantos K, Meimeth‐Damianaki T, Vaxevanoglou T, Kapetanakis E. Prospective study of a modified gonadotropin‐releasing hormone agonist long protocol in an in vitro fertilization program. Fertility and Sterility 1994;61(4):709‐13. [PUBMED: 8150115] - PubMed
Remorgida 1989 {published data only}
-
- Remorgida V, Anserini P, Croce S, Costa M, Ferraiolo A, Centonze A, et al. The duration of pituitary suppression by means of intranasal gonadotropin hormone‐releasing hormone analogue administration does not influence the ovarian response to gonadotropin stimulation and success rate in a gamete intrafallopian transfer (GIFT) program. Journal of In Vitro Fertilization and Embryo Transfer 1989;6(2):76‐80. [PUBMED: 2498446] - PubMed
Rodrigues 2014 {published data only}
Ron‐El 1992 {published data only}
-
- Ron‐El R, Herman A, Golan A, Soffer Y, Nachum H, Caspi E. Ultrashort gonadotropin‐releasing hormone agonist (GnRH‐a) protocol in comparison with the long‐acting GnRH‐a protocol and menotropin alone. Fertility and Sterility 1992;58(6):1164‐8. [PUBMED: 1459267] - PubMed
Sarhan 2012 {published data only}
-
- Sarhan A, Elsamanoudy A, Harira M. Hormonal responses and clinical outcome are the same with three doses of gonadotropin‐releasing hormone agonist used in short stimulation protocol. Human Reproduction 2012;27 (Suppl 2):ii302‐ii337. - PubMed
Sathanandan 1989 {published data only}
-
- Sathanandan M, Warnes GM, Kirby CA, Petrucco OM, Matthews CD. Adjuvant leuprolide in normal, abnormal, and poor responders to controlled ovarian hyperstimulation for in vitro fertilization/gamete intrafallopian transfer. Fertility and Sterility 1989;51(6):998‐1006. [PUBMED: 2498135] - PubMed
Smitz 1992 {published data only}
-
- Smitz J, Abbeel E, Bollen N, Camus M, Devroy P, Tournaye H, et al. The effect of gonadotrophin‐releasing hormone (GnRH) agonist in the follicular phase on in‐vitro fertilization outcome in normo‐ovulatory women. Human Reproduction (Oxford, England) 1992;7(8):1098‐102. [PUBMED: 1400933] - PubMed
Smitz 1992a {published data only}
-
- Smitz J, Erard P, Camus M, Devroey P, Tournaye H, Wisanto A, Steirteghem AC. Pituitary gonadotrophin secretory capacity during the luteal phase in superovulation using GnRH‐agonists and HMG in a desensitization or flare‐up protocol. Human Reproduction (Oxford, England) 1992;7(9):1225‐9. [PUBMED: 1479002] - PubMed
Stenbæk 2013 {published data only}
-
- Stenbæk DS, Toftager M, Hjordt LV, Jensen PS, Holst K, Holland T, et al. A comparison of changes in mental distress in women undergoing short versus long in vitro fertilization protocol: a clinical prospective randomized trial. Human Reproduction 2013;28(Suppl):i261‐i282.
Suganuma 1996 {published data only}
-
- Suganuma N, Tsukahara SI, Kitagawa T, Furuhashi M, Asada Y, Kondo I. A controlled ovarian hyperstimulation regimen involving intermittent gonadotropin administration with a "short" protocol of gonadotropin releasing hormone agonist for in vitro fertilization. Journal of Assisted Reproduction and Genetics 1996;13(1):43‐8. [PUBMED: 8825166] - PubMed
Tanaka 2014 {published data only}
-
- Tanaka A, Nagayoshi M, Tanaka I. Selection of an optimal controlled ovarian hyperstimulation method in relation to the number of antral follicles in patients less than 40 years old. Fertility and Sterility 2014;102(3):e223.
Tarin 1990 {published data only}
-
- Tarin JJ, Pellicer A. Consequences of high ovarian response to gonadotropins: a cytogenetic analysis of unfertilized human oocytes. Fertility and Sterility 1990;54(4):665‐70. [PUBMED: 2209887] - PubMed
Tarlatzis 1993 {published data only}
-
- Tarlatzis BC, Pados G, Bontis J, Lagos S, Grimbizis G, Spanos E, et al. Ovarian stimulation with Buserelin/HMG/HCG: prospective randomized study of short versus long protocol. Human Reproduction (Oxford, England) 1993;8(6):807‐12. [PUBMED: 8345067] - PubMed
Tarlatzis 1994 {published data only}
-
- Tarlatzis BC, Grimbizis G, Pournaropoulos F, Bontis J, Lagos S, Pados G, et al. Evaluation of two gonadotropin‐releasing hormone (GnRH) analogues (leuprolide and buserelin) in short and long protocols for assisted reproduction techniques. Journal of Assisted Reproduction and Genetics 1994;11(2):85‐91. [PUBMED: 7819707] - PubMed
Tehraninejad 2010 {published data only}
-
- Tehraninejad ESh, Nekoo EA, Ezabadi Z, Rashidi BH, Amirchaghmaghi E, Matroud EP. Half‐dose, long‐acting gonadotropin‐releasing hormone agonist (Diphereline) is comparable with daily injections of short‐acting gonadotropin‐releasing hormone agonist (Suprefact) in IVF/ICSI cycles. Archives of Medical Science 2010;6(6):945‐9. [PUBMED: 22427771] - PMC - PubMed
van de‐Helder 1990b {published data only}
-
- de‐Helder AB, Helmerhorst FM, Blankhart A, Brand R, Waegemaekers C, Naaktgeboren N. Comparison of ovarian stimulation regimens for in vitro fertilization (IVE) with and without a gonadotropin‐releasing hormone (GnRH) agonist: results of a randomized study. Journal of In Vitro Fertilization and Embryo Transfer 1990;7(6):358‐62. [PUBMED: 2127604] - PubMed
Wu 2012 {published data only}
-
- Wu Z, Li R, Ma Y, Deng B, Zhang X, Meng Y, et al. Effect of HCG‐day serum progesterone and oestradiol concentrations on pregnancy outcomes in GnRH agonist cycles. Reproductive Biomedicine Online 2012;24(5):511‐20. [PUBMED: 22417667] - PubMed
Yang 1991 {published data only}
-
- Yang YS, Ho HN, Lien YR, Hwang JL, Melinda S, Lin HR, et al. The use of a long‐acting gonadotropin‐releasing hormone analog (D‐Trp‐6‐LHRH) for improvement of ovarian stimulation in assisted conception programs. Journal of the Formosan Medical Association 1991;90(11):1081‐5. [PUBMED: 1687055] - PubMed
References to ongoing studies
NCT01006954 {published data only}
-
- NCT01006954. Comparison of micro dose gonadotropin‐releasing hormone (GnRH) agonist flare up & flare protocol in poor responders in assisted reproductive technology (ART) cycle. clinicaltrials.gov/ct2/show/NCT01006954 (accessed 04/2015).
Additional references
Barlow 1998
-
- Barlow DH. GnRHagonists and in vitro fertilization. Journal of Reproductive Medicine 1998;43 Suppl:245‐51. - PubMed
Donderwinkel 1993
-
- Donderwinkel PF, Schoot DC, Pache TD, Jong FH, Hop WC, Fauser BC. Luteal function following ovulation induction in polycystic ovary syndrome patients using exogenous gonadotrophins in combination with a gonadotrophin‐releasing hormone agonist. Human Reproduction (Oxford, England) 1993;8(12):2027‐32. [PUBMED: 8150898] - PubMed
Fields 2013
Griffin 2002
-
- Griffin PD, Rowe PJ, Vayena E, editors. Current practices and controversies in assisted reproduction: report of a WHO meeting on "Medical, Ethical and Social Aspects of Assisted Reproduction". Geneva, Switzerland: World Health Organization, 2002. [ISBN 92 4 159030 0]
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [ updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hughes 1992
-
- Hughes EG, Fedorkow DM, Daya S, Sagle MA, Koppel P, Collins JA. The routine use of gonadotropin‐releasing hormone agonists prior to in vitro fertilization and gamete intrafallopian transfer: a meta‐analysis of randomized controlled trials. Fertility and Sterility 1992;58(5):888‐96. [PUBMED: 1426372] - PubMed
Jenkins 1996
-
- Jenkins JM. The influence, development and management of functional ovarian cysts during IVF cycles. Journal of the British Fertility Society 1996;1:132‐6.
Maheshwari 2008
-
- Maheshwari A, Hamilton M, Bhattacharya S. Effect of female age on the diagnostic categories of infertility. Human Reproduction (Oxford, England) 2008;23(3):538‐42. [PUBMED: 18308834] - PubMed
Mancini 2011
-
- Mancini F, Tur R, Martinez F, Coroleu B, Rodríguez I, Barri PN. Gonadotrophin‐releasing hormone‐antagonists vs long agonist in in‐vitro fertilization patients with polycystic ovary syndrome: a meta‐analysis. Gynecological Endocrinology: the Official Journal of the International Society of Gynecological Endocrinology 2011;27(3):150‐5. [PUBMED: 21117862] - PubMed
Min 2004
-
- Min JK, Breheny SA, Maclachlan V, Healy DL. What is the most relevant standard of success in assisted reproduction? The singleton, term gestation, live birth rate per cycle initiated: the BESST endpoint for assisted reproduction. Human Reproduction (Oxford, England) 2004;19(1):3‐7. [PUBMED: 14688149] - PubMed
Mohsen 2013
-
- Mohsen IA, Din RE. Minimal stimulation protocol using letrozole versus microdose flare up GnRH agonist protocol in women with poor ovarian response undergoing ICSI. Gynecological Endocrinology: The Official Journal of the International Society of Gynecological Endocrinology 2013;29(2):105‐8. [PUBMED: 23134528] - PubMed
Regan 1990
-
- Regan L, Owen EJ, Jacobs HS. Hypersecretion of luteinising hormone, infertility, and miscarriage. Lancet 1990;336(8724):1141‐4. [PUBMED: 1978024] - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Stuck 1998
Sungurtekin 1995
-
- Sungurtekin U, Jansen RP. Profound luteinizing hormone suppression after stopping the gonadotropin‐releasing hormone‐agonist leuprolide acetate. Fertility and Sterility 1995;63(3):663‐5. [PUBMED: 7851604] - PubMed
Westergaard 2000
-
- Westergaard LG, Laursen SB, Andersen CY. Increased risk of early pregnancy loss by profound suppression of luteinizing hormone during ovarian stimulation in normogonadotrophic women undergoing assisted reproduction. Human Reproduction (Oxford, England) 2000;15(5):1003‐8. [PUBMED: 10783342] - PubMed
References to other published versions of this review
Daya 2000
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

