CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition
- PMID: 26558876
- PMCID: PMC4892769
- DOI: 10.1097/COC.0000000000000239
CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition
Abstract
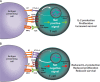
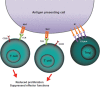
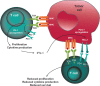
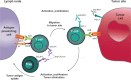
The cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed death 1 (PD-1) immune checkpoints are negative regulators of T-cell immune function. Inhibition of these targets, resulting in increased activation of the immune system, has led to new immunotherapies for melanoma, non-small cell lung cancer, and other cancers. Ipilimumab, an inhibitor of CTLA-4, is approved for the treatment of advanced or unresectable melanoma. Nivolumab and pembrolizumab, both PD-1 inhibitors, are approved to treat patients with advanced or metastatic melanoma and patients with metastatic, refractory non-small cell lung cancer. In addition the combination of ipilimumab and nivolumab has been approved in patients with BRAF WT metastatic or unresectable melanoma. The roles of CTLA-4 and PD-1 in inhibiting immune responses, including antitumor responses, are largely distinct. CTLA-4 is thought to regulate T-cell proliferation early in an immune response, primarily in lymph nodes, whereas PD-1 suppresses T cells later in an immune response, primarily in peripheral tissues. The clinical profiles of immuno-oncology agents inhibiting these 2 checkpoints may vary based on their mechanistic differences. This article provides an overview of the CTLA-4 and PD-1 pathways and implications of their inhibition in cancer therapy.
Conflict of interest statement
E.I.B. received research funding from Bristol-Myers Squibb, Genentech, and Merck; spouse previously employed by Merck. A.D. declares no conflicts of interest.
Figures
References
-
- Goldrath AW, Bevan MJ. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999;402:255–262. - PubMed
-
- Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–182. - PubMed
-
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. - PubMed
-
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

