In vivo epigenetic effects induced by engineered nanomaterials: A case study of copper oxide and laser printer-emitted engineered nanoparticles
- PMID: 26559097
- PMCID: PMC4958020
- DOI: 10.3109/17435390.2015.1108473
In vivo epigenetic effects induced by engineered nanomaterials: A case study of copper oxide and laser printer-emitted engineered nanoparticles
Abstract
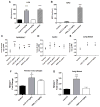
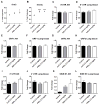
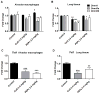
Evidence continues to grow on potential environmental health hazards associated with engineered nanomaterials (ENMs). While the geno- and cytotoxic effects of ENMs have been investigated, their potential to target the epigenome remains largely unknown. The aim of this study is two-fold: 1) determining whether or not industry relevant ENMs can affect the epigenome in vivo and 2) validating a recently developed in vitro epigenetic screening platform for inhaled ENMs. Laser printer-emitted engineered nanoparticles (PEPs) released from nano-enabled toners during consumer use and copper oxide (CuO) were chosen since these particles induced significant epigenetic changes in a recent in vitro companion study. In this study, the epigenetic alterations in lung tissue, alveolar macrophages and peripheral blood from intratracheally instilled mice were evaluated. The methylation of global DNA and transposable elements (TEs), the expression of the DNA methylation machinery and TEs, in addition to general toxicological effects in the lung were assessed. CuO exhibited higher cell-damaging potential to the lung, while PEPs showed a greater ability to target the epigenome. Alterations in the methylation status of global DNA and TEs, and expression of TEs and DNA machinery in mouse lung were observed after exposure to CuO and PEPs. Additionally, epigenetic changes were detected in the peripheral blood after PEPs exposure. Altogether, CuO and PEPs can induce epigenetic alterations in a mouse experimental model, which in turn confirms that the recently developed in vitro epigenetic platform using macrophage and epithelial cell lines can be successfully utilized in the epigenetic screening of ENMs.
Keywords: Copper oxide; DNA methylation; epigenetics; printer-emitted engineered nanoparticles; transposable elements.
Figures

Similar articles
-
Short-term exposure to engineered nanomaterials affects cellular epigenome.Nanotoxicology. 2016;10(2):140-50. doi: 10.3109/17435390.2015.1025115. Epub 2015 May 4. Nanotoxicology. 2016. PMID: 25938281 Free PMC article.
-
Effects of Laser Printer-Emitted Engineered Nanoparticles on Cytotoxicity, Chemokine Expression, Reactive Oxygen Species, DNA Methylation, and DNA Damage: A Comprehensive in Vitro Analysis in Human Small Airway Epithelial Cells, Macrophages, and Lymphoblasts.Environ Health Perspect. 2016 Feb;124(2):210-9. doi: 10.1289/ehp.1409582. Epub 2015 Jun 16. Environ Health Perspect. 2016. PMID: 26080392 Free PMC article.
-
Consumer exposures to laser printer-emitted engineered nanoparticles: A case study of life-cycle implications from nano-enabled products.Nanotoxicology. 2015;9(6):760-8. doi: 10.3109/17435390.2014.976602. Epub 2014 Nov 11. Nanotoxicology. 2015. PMID: 25387251 Free PMC article.
-
The effect of exposure to nanoparticles and nanomaterials on the mammalian epigenome.Int J Nanomedicine. 2016 Nov 25;11:6297-6306. doi: 10.2147/IJN.S120104. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27932878 Free PMC article. Review.
-
Can CuO nanoparticles lead to epigenetic regulation of antioxidant enzyme system?J Appl Toxicol. 2017 Jan;37(1):84-91. doi: 10.1002/jat.3392. Epub 2016 Sep 30. J Appl Toxicol. 2017. PMID: 27687502 Review.
Cited by
-
Silver, Gold, and Iron Oxide Nanoparticles Alter miRNA Expression but Do Not Affect DNA Methylation in HepG2 Cells.Materials (Basel). 2019 Mar 29;12(7):1038. doi: 10.3390/ma12071038. Materials (Basel). 2019. PMID: 30934809 Free PMC article.
-
Epigenetic Effects of Nanomaterials and Nanoparticles.J Nanobiotechnology. 2021 Jan 6;19(1):2. doi: 10.1186/s12951-020-00740-0. J Nanobiotechnology. 2021. PMID: 33407537 Free PMC article. Review.
-
Mutuality of epigenetic and nanoparticles: two sides of a coin.Heliyon. 2023 Dec 13;10(1):e23679. doi: 10.1016/j.heliyon.2023.e23679. eCollection 2024 Jan 15. Heliyon. 2023. PMID: 38187314 Free PMC article. Review.
-
Inactivation of common hospital acquired pathogens on surfaces and in air utilizing engineered water nanostructures (EWNS) based nano-sanitizers.Nanomedicine. 2019 Jun;18:234-242. doi: 10.1016/j.nano.2019.03.003. Epub 2019 Mar 20. Nanomedicine. 2019. PMID: 30904585 Free PMC article.
-
Evaluation of the cytotoxic and cellular proteome impacts of food-grade TiO2 (E171) using simulated gastrointestinal digestions and a tri-culture small intestinal epithelial model.NanoImpact. 2020 Jan;17:10.1016/j.impact.2019.100202. doi: 10.1016/j.impact.2019.100202. NanoImpact. 2020. PMID: 32133427 Free PMC article.
References
-
- Ahamed M, Akhtar MJ, Alhadlaq HA, Alrokayan SA. Assessment of the lung toxicity of copper oxide nanoparticles: current status. Nanomedicine (Lond) 2015;10:2365–2377. - PubMed
-
- Beck BD, Brain JD, Bohannon DE. An in vivo hamster bioassay to assess the toxicity of particulates for the lungs. Toxicol Appl Pharmacol. 1982;66:9–29. - PubMed
-
- Bello D, Martin J, Santeufemio C, Sun Q, Lee Bunker K, Shafer M, et al. Physicochemical and morphological characterisation of nanoparticles from photocopiers: implications for environmental health. Nanotoxicology. 2013;7:989–1003. - PubMed
-
- Bourc’his D, Bestor TH. Meiotic catastrophe and retrotransposon reactivation in male germ cells lacking Dnmt3L. Nature. 2004;431:96–99. - PubMed
-
- Chia SL, Tay CY, Setyawati MI, Leong DT. Biomimicry 3D gastrointestinal spheroid platform for the assessment of toxicity and inflammatory effects of zinc oxide nanoparticles. Small. 2015;11:702–712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
