Computerised cognitive behaviour therapy (cCBT) as treatment for depression in primary care (REEACT trial): large scale pragmatic randomised controlled trial
- PMID: 26559241
- PMCID: PMC4641883
- DOI: 10.1136/bmj.h5627
Computerised cognitive behaviour therapy (cCBT) as treatment for depression in primary care (REEACT trial): large scale pragmatic randomised controlled trial
Erratum in
-
Computerised cognitive behaviour therapy (cCBT) as treatment for depression in primary care (REEACT trial): large scale pragmatic randomised controlled trial.BMJ. 2016 Jan 12;352:i195. doi: 10.1136/bmj.i195. BMJ. 2016. PMID: 26759375 Free PMC article. No abstract available.
Abstract
Study question: How effective is supported computerised cognitive behaviour therapy (cCBT) as an adjunct to usual primary care for adults with depression?
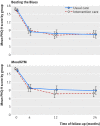
Methods: This was a pragmatic, multicentre, three arm, parallel randomised controlled trial with simple randomisation. Treatment allocation was not blinded. Participants were adults with symptoms of depression (score ≥ 10 on nine item patient health questionnaire, PHQ-9) who were randomised to receive a commercially produced cCBT programme ("Beating the Blues") or a free to use cCBT programme (MoodGYM) in addition to usual GP care. Participants were supported and encouraged to complete the programme via weekly telephone calls. Control participants were offered usual GP care, with no constraints on the range of treatments that could be accessed. The primary outcome was severity of depression assessed with the PHQ-9 at four months. Secondary outcomes included health related quality of life (measured by SF-36) and psychological wellbeing (measured by CORE-OM) at four, 12, and 24 months and depression at 12 and 24 months.
Study answer and limitations: Participants offered commercial or free to use cCBT experienced no additional improvement in depression compared with usual GP care at four months (odds ratio 1.19 (95% confidence interval 0.75 to 1.88) for Beating the Blues v usual GP care; 0.98 (0.62 to 1.56) for MoodGYM v usual GP care). There was no evidence of an overall difference between either programme compared with usual GP care (0.99 (0.57 to 1.70) and 0.68 (0.42 to 1.10), respectively) at any time point. Commercially provided cCBT conferred no additional benefit over free to use cCBT or usual GP care at any follow-up point. Uptake and use of cCBT was low, despite regular telephone support. Nearly a quarter of participants (24%) had dropped out by four months. The study did not have enough power to detect small differences so these cannot be ruled out. Findings cannot be generalised to cCBT offered with a much higher level of guidance and support.
What this study adds: Supported cCBT does not substantially improve depression outcomes compared with usual GP care alone. In this study, neither a commercially available nor free to use computerised CBT intervention was superior to usual GP care.
Funding, competing interests, data sharing: Commissioned and funded by the UK National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme (project No 06/43/05). The authors have no competing interests. Requests for patient level data will be considered by the REEACT trial management groupTrial registration Current Controlled Trials ISRCTN91947481.
© Gilbody et al 2015.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures
Comment in
-
Computerised self help for depression in primary care.BMJ. 2015 Nov 11;351:h5942. doi: 10.1136/bmj.h5942. BMJ. 2015. PMID: 26561033 No abstract available.
Similar articles
-
A randomised controlled trial of computerised cognitive behaviour therapy for the treatment of depression in primary care: the Randomised Evaluation of the Effectiveness and Acceptability of Computerised Therapy (REEACT) trial.Health Technol Assess. 2015 Dec;19(101):viii, xxi-171. doi: 10.3310/hta191010. Health Technol Assess. 2015. PMID: 26685904 Free PMC article. Clinical Trial.
-
The second Randomised Evaluation of the Effectiveness, cost-effectiveness and Acceptability of Computerised Therapy (REEACT-2) trial: does the provision of telephone support enhance the effectiveness of computer-delivered cognitive behaviour therapy? A randomised controlled trial.Health Technol Assess. 2016 Nov;20(89):1-64. doi: 10.3310/hta20890. Health Technol Assess. 2016. PMID: 27922448 Free PMC article. Clinical Trial.
-
Cost-effectiveness of computerized cognitive-behavioural therapy for the treatment of depression in primary care: findings from the Randomised Evaluation of the Effectiveness and Acceptability of Computerised Therapy (REEACT) trial.Psychol Med. 2017 Jul;47(10):1825-1835. doi: 10.1017/S0033291717000289. Epub 2017 Feb 23. Psychol Med. 2017. PMID: 28228182 Clinical Trial.
-
Improving the Effectiveness of Psychological Interventions for Depression and Anxiety in Cardiac Rehabilitation: The PATHWAY Research Programme Including 4 RCTs.Southampton (UK): National Institute for Health and Care Research; 2024 Sep. Southampton (UK): National Institute for Health and Care Research; 2024 Sep. PMID: 39353053 Free Books & Documents. Review.
-
Behavioural modification interventions for medically unexplained symptoms in primary care: systematic reviews and economic evaluation.Health Technol Assess. 2020 Sep;24(46):1-490. doi: 10.3310/hta24460. Health Technol Assess. 2020. PMID: 32975190 Free PMC article.
Cited by
-
[Efficacy of internet-based interventions for depression available in Germany-A systematic review and meta-analysis].Nervenarzt. 2024 Mar;95(3):206-215. doi: 10.1007/s00115-023-01587-0. Epub 2024 Jan 23. Nervenarzt. 2024. PMID: 38260995 Free PMC article. German.
-
Evaluation of the impact of a digital care navigator on increasing patient registration with digital mental health interventions in routine care.Internet Interv. 2024 Sep 17;38:100777. doi: 10.1016/j.invent.2024.100777. eCollection 2024 Dec. Internet Interv. 2024. PMID: 39410952 Free PMC article.
-
Evaluating the Therapeutic Alliance With a Free-Text CBT Conversational Agent (Wysa): A Mixed-Methods Study.Front Digit Health. 2022 Apr 11;4:847991. doi: 10.3389/fdgth.2022.847991. eCollection 2022. Front Digit Health. 2022. PMID: 35480848 Free PMC article.
-
Study protocol for a factorial-randomized controlled trial evaluating the implementation, costs, effectiveness, and sustainment of digital therapeutics for substance use disorder in primary care (DIGITS Trial).Implement Sci. 2023 Feb 1;18(1):3. doi: 10.1186/s13012-022-01258-9. Implement Sci. 2023. PMID: 36726127 Free PMC article.
-
Care Managers and Role Ambiguity: The Challenges of Supporting the Mental Health Needs of Patients with Chronic Conditions.Comput Support Coop Work. 2021 Feb;30(1):1-34. doi: 10.1007/s10606-020-09391-z. Epub 2021 Jan 18. Comput Support Coop Work. 2021. PMID: 34149187 Free PMC article.
References
-
- Üstün T, Ayuso-Mateos JL, Chatterji S, Mathers C, Murray CJ. Global burden of depressive disorders in the year 2000. Br J Psychiatry 2004;184:386-92. - PubMed
-
- Lester H, Howe A. Depression in primary care: three key challenges. Postgrad Med J 2008;84:545-8. - PubMed
-
- Roth A, Fonagy P. What works for whom? A critical review of psychotherapy research. Guilford Press, 2005.
-
- National Institute for Health and Care Excellence. Depression in adults: the treatment and management of depression in adults (Update-NICE clinical guideline 90). NICE, 2009.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
