Paradoxical Autoinflammatory Skin Reaction to Tumor Necrosis Factor Alpha Blockers Manifesting as Amicrobial Pustulosis of the Folds in Patients With Inflammatory Bowel Diseases
- PMID: 26559252
- PMCID: PMC4912246
- DOI: 10.1097/MD.0000000000001818
Paradoxical Autoinflammatory Skin Reaction to Tumor Necrosis Factor Alpha Blockers Manifesting as Amicrobial Pustulosis of the Folds in Patients With Inflammatory Bowel Diseases
Abstract
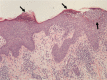
The therapy of inflammatory bowel disease, particularly with tumor necrosis factor (TNF) blockers, may be associated with a number of cutaneous adverse effects, including psoriasis-like, eczema-like, and lichenoid eruptions. Other rare skin complications are neutrophilic dermatoses such as amicrobial pustulosis of the folds (APF), which is a chronic relapsing pustular disorder classified in this spectrum.The authors analyzed clinical, histopathologic, and cytokine expression profiles of 3 inflammatory bowel disease patients with APF triggered by adalimumab (patient 1) and infliximab (patients 2 and 3).All 3 patients presented with sterile pustules involving the cutaneous folds, genital regions, and scalp 6 months after starting adalimumab (patient 1) and 9 months after starting infliximab (patients 2 and 3). Histology was characterized by epidermal spongiform pustules with a dermal neutrophilic and lymphocytic infiltrate. Tumor necrosis factor blocker withdrawal associated with topical and systemic corticosteroids induced complete remission of APF in all 3 patients. The expressions of interleukin (IL)-1 beta and its receptors as well as TNF alpha and its receptors were significantly higher in APF than in controls. Also IL-17, leukocyte selectin, and chemokines, such as IL-8, [C-X-C motif] chemokine ligand 1/2/3 (C = cysteine, X = any amino acid), [C-X-C motif] chemokine ligand 16 (C = cysteine, X = any amino acid), and RANTES (regulated on activation, normal T cell expressed and secreted) were significantly overexpressed. Finally, the authors found significant overexpression of both metalloproteinases 2/9 and their inhibitors 1/2.The observation of 3 patients with APF following anti-TNF therapy expands not only the clinical context of APF but also the spectrum of anti-TNF side effects. Overexpression of cytokines/chemokines and molecules amplifying the inflammatory network supports the view that APF is autoinflammatory in origin.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures







References
-
- Burgdorf W. Cutaneous manifestations of Crohn's disease. J Am Acad Dermatol 1981; 5:689–695. - PubMed
-
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn's disease: a review. J Eur Acad Dermatol Venereol 2008; 22:1033–1043. - PubMed
-
- Timani S, Mutasim DF. Skin manifestations of inflammatory bowel disease. Clin Dermatol 2008; 26:265–273. - PubMed
-
- Marzano AV, Borghi A, Stadnicki A, et al. Cutaneous manifestations in patients with inflammatory bowel diseases: pathophysiology, clinical features, and therapy. Inflamm Bowel Dis 2014; 20:213–227. - PubMed

