Association Between Clinically Staged Node-Negative Esophageal Adenocarcinoma and Overall Survival Benefit From Neoadjuvant Chemoradiation
- PMID: 26559488
- PMCID: PMC5340565
- DOI: 10.1001/jamasurg.2015.4068
Association Between Clinically Staged Node-Negative Esophageal Adenocarcinoma and Overall Survival Benefit From Neoadjuvant Chemoradiation
Abstract
Importance: While neoadjuvant chemoradiation for esophageal cancer improves oncologic outcomes for a broad group of patients with locally advanced and/or node-positive tumors, it is less clear which specific subset of patients derives most benefit in terms of overall survival (OS).
Objective: To determine whether neoadjuvant chemoradiation based on esophageal adenocarcinoma histology has similar oncologic outcomes for patients treated with surgery alone when stratified by clinical nodal status.
Design, setting, and participants: A retrospective analysis using the American College of Surgeons National Cancer Database from 1998 to 2006. Patients with esophageal adenocarcinoma histology and clinical stage T1bN1-N3 or T2-T4aN-/+M0 were divided into 2 treatment groups: (1) neoadjuvant chemoradiation followed by surgery and (2) surgery alone. Subset analysis within each treatment group was performed for clinically node-negative patients (cN-) vs node-positive patients (cN+) in conjunction with pathological nodal status. A propensity score-adjusted analysis, which included patient demographics, comorbidity status, and clinical T stage, was also performed.
Main outcome and measures: The primary outcome was 3-year OS. Secondary outcomes included margin status, postoperative length of stay, unplanned readmission rate, and 30-day mortality.
Results: A total of 1309 patients were identified, of whom 539 received neoadjuvant chemoradiation followed by surgery and 770 received surgery alone. Of the 1309 patients, 41.2% (n = 539) received neoadjuvant chemoradiation and 47.2% (n = 618) were cN+. Median follow-up for the entire cohort was 73.3 months (interquartile range, 64.1-93.5 months). The 3-year OS was better for neoadjuvant chemoradiation followed by surgery compared with surgery alone (49% vs 38%, respectively; P < .001). Stratifying based on clinical nodal status, the propensity score-adjusted OS was significantly better for cN+ patients who received neoadjuvant chemoradiation (hazard ratio, 0.52; 95% CI, 0.42-0.66; P < .001). In contrast, there was no difference in OS for cN- patients based on treatment (hazard ratio, 0.84; 95% CI, 0.65-1.10; P = .22).
Conclusions and relevance: Patients with cN+ esophageal adenocarcinoma benefit significantly from neoadjuvant chemoradiation. However, patients with cN- tumors treated with neoadjuvant chemoradiation plus surgery do not derive a significant OS benefit compared with surgery alone. This finding may have significant implications on the use of neoadjuvant chemoradiation in patients with cN- disease.
Conflict of interest statement
Figures

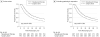

Comment in
-
Preoperative Chemoradiation in an Era of Suboptimal Clinical Staging.JAMA Surg. 2016 Mar;151(3):245-6. doi: 10.1001/jamasurg.2015.4047. JAMA Surg. 2016. PMID: 26558532 No abstract available.
Similar articles
-
Utility of Adjuvant Chemotherapy After Neoadjuvant Chemoradiation and Esophagectomy for Esophageal Cancer.Ann Surg. 2017 Aug;266(2):297-304. doi: 10.1097/SLA.0000000000001954. Ann Surg. 2017. PMID: 27501170
-
Survival in Patients With Esophageal Adenocarcinoma Undergoing Trimodality Therapy Is Independent of Regional Lymph Node Location.Ann Thorac Surg. 2016 Mar;101(3):1075-80; Discussion 1080-1. doi: 10.1016/j.athoracsur.2015.09.063. Epub 2015 Dec 8. Ann Thorac Surg. 2016. PMID: 26680311
-
Esophagectomy Timing After Neoadjuvant Therapy for Distal Esophageal Adenocarcinoma.Ann Thorac Surg. 2016 Mar;101(3):1123-30. doi: 10.1016/j.athoracsur.2015.09.044. Epub 2015 Dec 1. Ann Thorac Surg. 2016. PMID: 26652139
-
Optimal Use of Combined Modality Therapy in the Treatment of Esophageal Cancer.Surg Oncol Clin N Am. 2017 Jul;26(3):405-429. doi: 10.1016/j.soc.2017.01.009. Epub 2017 May 11. Surg Oncol Clin N Am. 2017. PMID: 28576180 Review.
-
Does timing of esophagectomy following neoadjuvant chemoradiation affect outcomes? A meta-analysis.Int J Surg. 2018 Nov;59:11-18. doi: 10.1016/j.ijsu.2018.09.013. Epub 2018 Sep 24. Int J Surg. 2018. PMID: 30261331 Review.
Cited by
-
Staging esophageal cancer: low EUS accuracy in t2n0 patients.Endosc Int Open. 2021 Mar;9(3):E313-E318. doi: 10.1055/a-1336-2505. Epub 2021 Feb 18. Endosc Int Open. 2021. PMID: 33655027 Free PMC article.
-
Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for clinical node-negative esophageal carcinoma.Thorac Cancer. 2020 Sep;11(9):2618-2629. doi: 10.1111/1759-7714.13586. Epub 2020 Aug 4. Thorac Cancer. 2020. PMID: 32755068 Free PMC article.
-
ASO Author Reflections: Radiomics-Based Prediction of Individual Lymph Node Metastatic Status in Esophageal Squamous Cell Carcinoma.Ann Surg Oncol. 2022 Dec;29(13):8127-8128. doi: 10.1245/s10434-022-12230-8. Epub 2022 Aug 6. Ann Surg Oncol. 2022. PMID: 35933539 No abstract available.
-
Pathologic Complete Response Is an Independent Predictor of Improved Survival Following Neoadjuvant Chemoradiation for Esophageal Adenocarcinoma.J Gastrointest Surg. 2016 Sep;20(9):1541-6. doi: 10.1007/s11605-016-3177-0. Epub 2016 Jun 3. J Gastrointest Surg. 2016. PMID: 27260525
-
Nomogram based on multimodal magnetic resonance combined with B7-H3mRNA for preoperative lymph node prediction in esophagus cancer.World J Clin Oncol. 2024 Mar 24;15(3):419-433. doi: 10.5306/wjco.v15.i3.419. World J Clin Oncol. 2024. PMID: 38576593 Free PMC article.
References
-
- National Comprehensive Cancer Network. [Accessed January 5, 2015];Esophagus: version 4. http://www.nccn.org/professionals/physician_gls/pdf/esophagus.pdf Published 2014.
-
- Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359(9319):1727–1733. - PubMed
-
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. - PubMed
-
- American Joint Committee on Cancer. Manual for Staging of Cancer. 5th. Philadelphia, PA: Lippincott Raven Publishers; 1997.
-
- American Joint Committee on Cancer. Manual for Staging of Cancer. 6th. Philadelphia, PA: Lippincott Raven Publishers; 2002.
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

