The Cellular Protein Complex Associated with a Transforming Region of E1A Contains c-MYC
- PMID: 26559831
- PMCID: PMC4702669
- DOI: 10.1128/JVI.02039-15
The Cellular Protein Complex Associated with a Transforming Region of E1A Contains c-MYC
Abstract
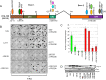
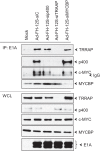
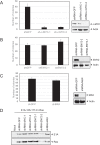
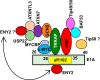
The cell-transforming activity of human adenovirus 5 (hAd5) E1A is mediated by the N-terminal half of E1A, which interacts with three different major cellular protein complexes, p300/CBP, TRRAP/p400, and pRb family members. Among these protein interactions, the interaction of pRb family proteins with conserved region 2 (CR2) of E1A is known to promote cell proliferation by deregulating the activities of E2F family transcription factors. The functional consequences of interaction with the other two protein complexes in regulating the transforming activity of E1A are not well defined. Here, we report that the E1A N-terminal region also interacted with the cellular proto-oncoprotein c-MYC and the homolog of enhancer of yellow 2 (ENY2). Our results suggested that these proteins interacted with an essential E1A transforming domain spanning amino acid residues 26 to 35 which also interacted with TRRAP and p400. Small interfering RNA (siRNA)-mediated depletion of TRRAP reduced c-MYC interaction with E1A, while p400 depletion did not. In contrast, depletion of TRRAP enhanced ENY2 interaction with E1A, suggesting that ENY2 and TRRAP may interact with E1A in a competitive manner. The same E1A region additionally interacted with the constituents of a deubiquitinase complex consisting of USP22, ATXN7, and ATXN7L3 via TRRAP. Acute short hairpin RNA (shRNA)-mediated depletion of c-MYC reduced the E1A transforming activity, while depletion of ENY2 and MAX did not. These results suggested that the association of c-MYC with E1A may, at least partially, play a role in the E1A transformation activity, independently of MAX.
Importance: The transforming region of adenovirus E1A consists of three short modules which complex with different cellular protein complexes. The mechanism by which one of the transforming modules, CR2, promotes cell proliferation, through inactivating the activities of the pRb family proteins, is better understood than the activities of the other domains. Our analysis of the E1A proteome revealed the presence of the proto-oncoprotein c-MYC and of ENY2. We mapped these interactions to a critical transforming module of E1A that was previously known to interact with the scaffolding molecule TRRAP and the E1A-binding protein p400. We showed that c-MYC interacted with E1A through TRRAP, while ENY2 interacted with it independently. The data reported here indicated that depletion of c-MYC in normal human cells reduced the transforming activity of E1A. Our result raises a novel paradigm in oncogenic transformation by a DNA viral oncogene, the E1A gene, that may exploit the activity of a cellular oncogene, the c-MYC gene, in addition to inactivation of the tumor suppressors, such as pRb.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
Ad E1A 243R oncoprotein promotes association of proto-oncogene product MYC with the NuA4/Tip60 complex via the E1A N-terminal repression domain.Virology. 2016 Dec;499:178-184. doi: 10.1016/j.virol.2016.09.005. Epub 2016 Sep 22. Virology. 2016. PMID: 27664947 Free PMC article.
-
Relationship between E1A binding to cellular proteins, c-myc activation and S-phase induction.Oncogene. 2007 Feb 1;26(5):781-7. doi: 10.1038/sj.onc.1209825. Epub 2006 Jul 24. Oncogene. 2007. PMID: 16862175
-
Recruitment of TRRAP required for oncogenic transformation by E1A.Oncogene. 2001 Dec 13;20(57):8270-5. doi: 10.1038/sj.onc.1205159. Oncogene. 2001. PMID: 11781841
-
Adenoviral E1A function through Myc.Cancer Res. 2009 Jan 1;69(1):6-9. doi: 10.1158/0008-5472.CAN-08-3026. Cancer Res. 2009. PMID: 19117980 Review.
-
Modulation of oncogenic transformation by the human adenovirus E1A C-terminal region.Curr Top Microbiol Immunol. 2004;273:139-61. doi: 10.1007/978-3-662-05599-1_5. Curr Top Microbiol Immunol. 2004. PMID: 14674601 Review.
Cited by
-
The adenoviral E1A N-terminal domain represses MYC transcription in human cancer cells by targeting both p300 and TRRAP and inhibiting MYC promoter acetylation of H3K18 and H4K16.Genes Cancer. 2016 Mar;7(3-4):98-109. doi: 10.18632/genesandcancer.99. Genes Cancer. 2016. PMID: 27382434 Free PMC article.
-
The Diarylheptanoid Curcumin Induces MYC Inhibition and Cross-Links This Oncoprotein to the Coactivator TRRAP.Front Oncol. 2021 Apr 15;11:660481. doi: 10.3389/fonc.2021.660481. eCollection 2021. Front Oncol. 2021. PMID: 33937075 Free PMC article.
-
Ad E1A 243R oncoprotein promotes association of proto-oncogene product MYC with the NuA4/Tip60 complex via the E1A N-terminal repression domain.Virology. 2016 Dec;499:178-184. doi: 10.1016/j.virol.2016.09.005. Epub 2016 Sep 22. Virology. 2016. PMID: 27664947 Free PMC article.
-
Adenovirus E1A binding to DCAF10 targets proteasomal degradation of RUVBL1/2 AAA+ ATPases required for quaternary assembly of multiprotein machines, innate immunity, and responses to metabolic stress.J Virol. 2023 Dec 21;97(12):e0099323. doi: 10.1128/jvi.00993-23. Epub 2023 Nov 14. J Virol. 2023. PMID: 37962355 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

