Trehalose, an mTOR-Independent Inducer of Autophagy, Inhibits Human Cytomegalovirus Infection in Multiple Cell Types
- PMID: 26559848
- PMCID: PMC4719619
- DOI: 10.1128/JVI.02651-15
Trehalose, an mTOR-Independent Inducer of Autophagy, Inhibits Human Cytomegalovirus Infection in Multiple Cell Types
Abstract
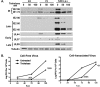
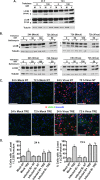
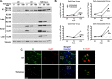
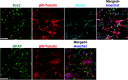
Human cytomegalovirus (HCMV) is the major viral cause of birth defects and a serious problem in immunocompromised individuals and has been associated with atherosclerosis. Previous studies have shown that the induction of autophagy can inhibit the replication of several different types of DNA and RNA viruses. The goal of the work presented here was to determine whether constitutive activation of autophagy would also block replication of HCMV. Most prior studies have used agents that induce autophagy via inhibition of the mTOR pathway. However, since HCMV infection alters the sensitivity of mTOR kinase-containing complexes to inhibitors, we sought an alternative method of inducing autophagy. We chose to use trehalose, a nontoxic naturally occurring disaccharide that is found in plants, insects, microorganisms, and invertebrates but not in mammals and that induces autophagy by an mTOR-independent mechanism. Given the many different cell targets of HCMV, we proceeded to determine whether trehalose would inhibit HCMV infection in human fibroblasts, aortic artery endothelial cells, and neural cells derived from human embryonic stem cells. We found that in all of these cell types, trehalose induces autophagy and inhibits HCMV gene expression and production of cell-free virus. Treatment of HCMV-infected neural cells with trehalose also inhibited production of cell-associated virus and partially blocked the reduction in neurite growth and cytomegaly. These results suggest that activation of autophagy by the natural sugar trehalose or other safe mTOR-independent agents might provide a novel therapeutic approach for treating HCMV disease.
Importance: HCMV infects multiple cell types in vivo, establishes lifelong persistence in the host, and can cause serious health problems for fetuses and immunocompromised individuals. HCMV, like all other persistent pathogens, has to finely tune its interplay with the host cellular machinery to replicate efficiently and evade detection by the immune system. In this study, we investigated whether modulation of autophagy, a host pathway necessary for the recycling of nutrients and removal of protein aggregates, misfolded proteins, and pathogens, could be used to target HCMV. We found that autophagy could be significantly increased by treatment with the nontoxic, natural disaccharide trehalose. Importantly, trehalose had a profound inhibitory effect on viral gene expression and strongly impaired viral spread. These data constitute a proof-of-concept for the use of natural products targeting host pathways rather than the virus itself, thus reducing the risk of the development of resistance to treatment.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures




Similar articles
-
Human Cytomegalovirus Replication Is Inhibited by the Autophagy-Inducing Compounds Trehalose and SMER28 through Distinctively Different Mechanisms.J Virol. 2018 Feb 26;92(6):e02015-17. doi: 10.1128/JVI.02015-17. Print 2018 Mar 15. J Virol. 2018. PMID: 29237845 Free PMC article.
-
Digitoxin Suppresses Human Cytomegalovirus Replication via Na+, K+/ATPase α1 Subunit-Dependent AMP-Activated Protein Kinase and Autophagy Activation.J Virol. 2018 Feb 26;92(6):e01861-17. doi: 10.1128/JVI.01861-17. Print 2018 Mar 15. J Virol. 2018. PMID: 29321306 Free PMC article.
-
Trehalose Inhibits Human Immunodeficiency Virus Type 1 Infection in Primary Human Macrophages and CD4+ T Lymphocytes through Two Distinct Mechanisms.J Virol. 2020 Aug 17;94(17):e00237-20. doi: 10.1128/JVI.00237-20. Print 2020 Aug 17. J Virol. 2020. PMID: 32554696 Free PMC article.
-
[Interrelationship between human cytomegalovirus infection and chemokine].Nihon Rinsho. 1998 Jan;56(1):69-74. Nihon Rinsho. 1998. PMID: 9465667 Review. Japanese.
-
Modulatory effects of human cytomegalovirus infection on malignant properties of cancer cells.Intervirology. 1996;39(4):259-69. doi: 10.1159/000150527. Intervirology. 1996. PMID: 9078467 Review.
Cited by
-
Effect of natural products on host cell autophagy induced by Influenza A virus infection.Front Cell Infect Microbiol. 2024 Sep 30;14:1460604. doi: 10.3389/fcimb.2024.1460604. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39403204 Free PMC article. Review.
-
Possible Therapeutic Use of Natural Compounds Against COVID-19.J Cell Signal. 2021;2(1):63-79. J Cell Signal. 2021. PMID: 33768214 Free PMC article.
-
Trehalose-Rich, Degradable Hydrogels Designed for Trehalose Release under Physiologically Relevant Conditions.Polymers (Basel). 2019 Dec 6;11(12):2027. doi: 10.3390/polym11122027. Polymers (Basel). 2019. PMID: 31817772 Free PMC article.
-
Essential role of protein kinase R antagonism by TRS1 in human cytomegalovirus replication.Virology. 2016 Feb;489:75-85. doi: 10.1016/j.virol.2015.11.032. Epub 2015 Dec 21. Virology. 2016. PMID: 26716879 Free PMC article.
-
Triptolide induces protective autophagy and apoptosis in human cervical cancer cells by downregulating Akt/mTOR activation.Oncol Lett. 2018 Sep;16(3):3929-3934. doi: 10.3892/ol.2018.9074. Epub 2018 Jul 4. Oncol Lett. 2018. PMID: 30128010 Free PMC article.
References
-
- Mocarski ES, Shenk T, Pass RF. 2007. Cytomegaloviruses, p 2701–2772. In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 5th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

