Cervical ripening before first trimester surgical evacuation for non-viable pregnancy
- PMID: 26559875
- PMCID: PMC9271321
- DOI: 10.1002/14651858.CD009954.pub2
Cervical ripening before first trimester surgical evacuation for non-viable pregnancy
Abstract
Background: Medications or mechanical dilators are often used to soften and dilate the cervix prior to surgical evacuation of the uterus for non-viable pregnancy, or miscarriage. The majority of miscarriages occur in the first trimester. The aim of cervical ripening is to reduce the possibility of injury to the uterus and cervix and improve the surgical ease of the procedure. Cervical ripening agents can have adverse effects and it is uncertain as to whether these risks outweigh the benefits of their use.
Objectives: To systematically review the benefits and harms of using cervical ripening agents prior to surgical evacuation of non-viable pregnancy prior to 14 weeks' gestation.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 April 2015) and reference lists of retrieved papers.
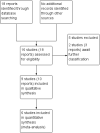
Selection criteria: Randomised controlled trials (published in full-text form, or as abstracts only), which assessed the use of pharmacological or mechanical agents to ripen the cervix in women undergoing dilation and curettage or vacuum aspiration for non-viable pregnancy at less than 14 weeks' gestation were eligible for inclusion. Cluster-randomised controlled trials and trials using a cross-over design were not eligible for inclusion.Unpublished randomised controlled trials and quasi-randomised trials would have been eligible for inclusion but none were identified.
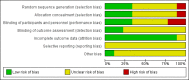
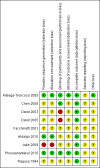
Data collection and analysis: Two review authors independently assessed the studies for inclusion, assessed risk of bias and carried out data extraction. Data were checked for accuracy.
Main results: We included nine trials with 469 women. A diverse set of medications and regimens were studied in these trials, making the comparisons available for meta-analysis limited. The comparisons draw data from six trials with 383 participants. All trials were relatively small and had several aspects of unclear risk of bias with few of this review's outcomes reported. Due to this, no data from three trials were able to be used despite them meeting inclusion criteria.We carried out four comparisons: isosorbide mononitrate or dinitrate compared with misoprostol; misoprostol compared with placebo; chemical dilation (use of medications) compared with mechanical dilation; and any cervical preparation compared with placebo.None of the included studies reported data on the review's primary outcome: cervical or uterine injury (perforation, laceration, creation of a false passage).No clear difference was shown between isosorbide compounds and misoprostol for the outcome need for manual cervical dilation (average risk ratio (RR) 0.76, 95% confidence interval (CI) 0.10 to 5.64; three trials, 150 women; Tau² = 2.11; I² = 69%), however the data were heterogenous. In terms of adverse effects, misoprostol was associated with more vomiting (RR 0.11, 95% CI 0.01 to 0.85; two trials, 120 women), however there were no clear differences between isosorbide compounds and misoprostol in relation to other reported adverse effects (headache, nausea or hypotension). The dosing regimens differed in terms of dose, number of administrations and route of administration in the different trials. Mechanical (Dilapan-S hygroscopic) dilators performed similarly to chemical dilators in a single trial (65 women) that measured difficulty in cervical dilation, excessive bleeding and adverse effects.Misoprostol was shown to be more effective than placebo for cervical ripening (reduced need for manual cervical dilation) (RR 0.14, 95% CI 0.08 to 0.26; one trial, 120 women), and surgical time was reduced when misoprostol was used (mean difference (MD) -3.15, 95% CI -3.59 to -2.70; one trial, 120 women). However, compared to placebo, misoprostol, was associated with more abdominal pain (RR 29.00, 95% CI 1.77 to 475.35; one trial, 120 women), although no clear differences in the risk of other adverse effects (nausea, vomiting, headache or fever) were observed between groups.There was no clear differences between chemical dilation and mechanical dilators for the outcomes: difficulty in cervical dilation, excessive bleeding or adverse effects.Compared with placebo, any cervical preparation reduced the need for manual cervical dilatation (average RR 0.25, 95% CI 0.07 to 0.89; two trials, 168 women; Tau² = 0.67; I² = 81%), and reduced surgical time (MD -2.55, 95% CI -3.67 to -1.43, two trials, 168 women; Tau² = 0.63; I² = 96%).None of the included trials reported on the review's other secondary outcomes, including: injury to bladder or bowel, miscarriage/preterm birth in a subsequent pregnancy, analgesia use after administration of ripening agent but before surgery, or analgesia use after surgery.
Authors' conclusions: This review found no evidence to evaluate cervical ripening prior to first trimester surgical evacuation for miscarriage for reducing the rate of cervical or uterine injury, however, this may be because these outcomes are very rare. Cervical preparation was shown to reduce the need for manual cervical dilatation compared with placebo.Misoprostol and isosorbide mononitrate and dinitrate were similarly effective in ripening the cervix, however there was more vomiting with misoprostol. Mechanical (Dilapan-S hygroscopic) dilators performed similarly to chemical dilators.The nine studies included in this review were small and the methodological quality of the trials was mixed, and for the most part, not well-described; thus any conclusions drawn from the data included in this review must be treated with caution. Consequently, large, high-quality trials are required to determine whether the benefits of this treatment outweigh the risks. Further research should be powered to assess the rate of cervical and uterine injury between interventions. Future research should also guide clinicians in deciding whether the benefits of reduced manual cervical dilatation outweigh the risks of adverse effects associated with these agents (nausea, vomiting, headache, fever, diarrhoea and pain). Women's satisfaction and outcomes of future pregnancies should also be assessed.
Conflict of interest statement
None known.
Figures























Update of
- doi: 10.1002/14651858.CD009954
Similar articles
-
Preoperative ripening of the cervix before operative hysteroscopy.Cochrane Database Syst Rev. 2015 Apr 23;2015(4):CD005998. doi: 10.1002/14651858.CD005998.pub2. Cochrane Database Syst Rev. 2015. PMID: 25906113 Free PMC article.
-
Methods for managing miscarriage: a network meta-analysis.Cochrane Database Syst Rev. 2021 Jun 1;6(6):CD012602. doi: 10.1002/14651858.CD012602.pub2. Cochrane Database Syst Rev. 2021. PMID: 34061352 Free PMC article.
-
Pharmacological and mechanical interventions for labour induction in outpatient settings.Cochrane Database Syst Rev. 2017 Sep 13;9(9):CD007701. doi: 10.1002/14651858.CD007701.pub3. Cochrane Database Syst Rev. 2017. PMID: 28901007 Free PMC article.
-
Nitric oxide donors for cervical ripening and induction of labour.Cochrane Database Syst Rev. 2016 Dec 5;12(12):CD006901. doi: 10.1002/14651858.CD006901.pub3. Cochrane Database Syst Rev. 2016. PMID: 27918616 Free PMC article.
-
Methods of term labour induction for women with a previous caesarean section.Cochrane Database Syst Rev. 2017 Jun 9;6(6):CD009792. doi: 10.1002/14651858.CD009792.pub3. Cochrane Database Syst Rev. 2017. PMID: 28599068 Free PMC article.
Cited by
-
Health care providers' perceptions of using misoprostol in the treatment of incomplete abortion in Malawi.BMC Health Serv Res. 2022 Dec 3;22(1):1471. doi: 10.1186/s12913-022-08878-3. BMC Health Serv Res. 2022. PMID: 36461125 Free PMC article.
References
References to studies included in this review
Arteaga‐Troncoso 2005 {published data only}
-
- Arteaga‐Troncoso G, Villegas‐Alvarado A, Belmont‐Gomez A, Martinez‐Herrera FJ, Villagrana‐Zesati R, Guerra‐Infante F. Intracervical application of the nitric oxide donor isosorbide dinitrate for induction of cervical ripening: a randomised controlled trial to determine clinical efficacy and safety prior to first trimester surgical evacuation of retained products of conception. BJOG: An International Journal of Obstetrics & Gynaecology 2005;112(12):1615‐9. - PubMed
Chen 2008 {published data only}
-
- Chen FC, Bergann A, Krosse J, Merholz A, David M. Isosorbide mononitrate vaginal gel versus misoprostol vaginal gel versus Dilapan‐S for cervical ripening before first trimester curettage. European Journal of Obstetrics & Gynecology and Reproductive Biology 2008;138(2):176‐9. - PubMed
David 2003 {published data only}
-
- David M, Chen FCK, Lichtenegger W. NO‐donor nitroglycerin versus the prostaglandin gemeprost for cervical ripening in first trimester missed abortion. International Journal of Gynecology & Obstetrics 2003;83(1):71‐2. - PubMed
David 2005 {published data only}
-
- David M, Chen FCK. Comparison of isosorbide mononitrate (Mono Mack) and misoprostol (Cytotec) for cervical ripening in the first trimester missed abortion. Archives of Gynecology and Obstetrics 2005;273(3):144‐5. - PubMed
Facchinetti 2001 {published data only}
-
- Facchinetti F, Piccinini F, Fazzio M, Volpe A. Safety and efficacy of nitroprusside gel for cervical ripening [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S107.
Hidalgo 2010 {published data only}
-
- Hidalgo M, Guerra M, Reyna E, Santos J, Mejia J, Reyna N, et al. Isosorbide mononitrate or misoprostol for cervical ripening in missed pregnancies during first trimester [Mononitrato de isosorbide o misoprostol en maduracion cervical en embarazos interrumpidos durante el primer trimestre]. Clinica e Investigacion en Ginecologia y Obstetricia 2010;37(6):218‐22.
Jabir 2009 {published data only}
-
- Jabir M, Smeet R. Comparison of oral and vaginal misoprostol for cervical ripening before evacuation of first trimester missed miscarriage. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S209. - PubMed
-
- Jabir M, Smeet RI. Comparison of oral and vaginal misoprostol for cervical ripening before evacuation of first trimester missed miscarriage. Saudi Medical Journal 2009;30(1):82‐7. - PubMed
Phusaanantakul 2010 {published data only}
-
- Phusaanantakul P, Promsonthi P, Chanrachakul B. Effect of isosorbide mononitrate for cervical ripening before surgical termination of pregnancy in the first trimester. International Journal of Gynecology & Obstetrics 2010;110(2):145‐8. - PubMed
Ragusa 1994 {published data only}
-
- Ragusa A, Vignali MJ, Zanetta G, Norchi S, Zanini A. Pre‐operative cervical preparation before first trimester missed abortion: a randomized controlled comparison between single or double intracervical administration of pge2 gel. Prostaglandins Leukotrienes & Essential Fatty Acids 1994;50(5):267‐9. - PubMed
References to studies excluded from this review
Dee 2009 {published data only}
-
- Dee MT, Dancel MAS. A prospective randomized study comparing the use of intrauterine to vaginal misoprostol for pre‐curettage cervical priming of early pregnancy failure. Philippine Journal of Obstetrics & Gynecology 2009;33(3):83.
Dunn 2008 {published data only}
-
- Dunn TS, Gregory N, Parrish A, Coddington III CC. Oral misoprostol compared with laminaria before dilation and curettage for missed abortion. Obstetrics & Gynecology 2008;111(4 Suppl):23S.
Firouzabadi 2012 {published data only}
-
- Firouzabadi RD, Sekhavat L, Tabatabaii A, Hamadani S. Laminaria tent versus Misoprostol for cervical ripening before surgical process in missed abortion. Archives of Gynecology and Obstetrics 2012;285(3):699‐703. - PubMed
Hernandez‐Valencia 2003 {published data only}
-
- Hernandez‐Valencia M. Cervical ripening with prostaglandin E1: how an ambulatory method decreases the hospital stay in abortus with intrauterine fetal demise. Fetal Diagnosis & Therapy 2003;18:54‐8. - PubMed
Surita 1997 {published data only}
-
- Surita FGC. Misoprostol versus laminaria for cervical ripening in intrauterine fetal death. Acta Obstetricia et Gynecologica Scandinavica Supplement 1997;76(167:2):32.
References to studies awaiting assessment
Marfou 2012 {published data only}
-
- Marfou J, Shahbazian N, Abdollahi M. Comparison of effect of oxytocin and prostaglandin E1 on cervix prior to curettage in first trimester. Iranian Journal of Obstetrics, Gynecology and Infertility 2012;15(28):9‐12.
Sripramote 2000 {published data only}
-
- Chatsuphang W, Sripramote M, Dejthevaporn T. A randomised comparison of oral and vaginal misoprostol for cervical priming before uterine curettage in the first trimester pregnancy. Thai Journal of Obstetrics and Gynaecology 2000; Vol. 12, issue 4:354.
-
- Sripramote M, Chatsuphang W. A randomized comparison of oral and vaginal misoprostol for cervical priming before uterine curettage in the first trimester of pregnancy. Vajira Medical Journal 2000;44(3):207‐15.
Additional references
Alberman 1992
-
- Alberman E. Spontaneous abortions: epidemiology. In: Stabile I, Grudzinskas G, Chard T editor(s). Spontaneous Abortion, Diagnosis and Treatment. London: Springer‐Verlag, 1992:11‐20.
Creinin 2001
-
- Creinin MD, Schwartz JL, Guido RS, Pymar HC. Early pregnancy failure‐‐current management concepts. Obstetrical and Gynecological Survey 2001;56:105‐13. - PubMed
Facchinetti 2000
-
- Facchinetti F, Piccinini F, Volpe A. Chemical ripening of the cervix with intracervical application of sodium nitroprusside: a randomised controlled trial. Human Reproduction 2000;15(10):2224‐7. - PubMed
Fiala 2007
-
- Fiala C, Gemzell‐Danielsson K, Tang OS, Hertzen H. Cervical priming with misoprostol prior to transcervical procedures. International Journal of Gynecology & Obstetrics 2007;99:S168‐S171. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kapp 2010
Kulier 2001
Kulier 2011
Lohr 2008
Martindale 2010
-
- Sweetman SC (editor). Martindale: the Complete Drug Reference. 4th Edition. London: Pharmaceutical Press, 2010.
May 2008
MIMS 2010 [Computer program]
-
- MIMS. MIMS online. Sydney, Australia: UBM Medica, 2010.
Newmann 2010
Renner 2009
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Say 2002
Tang 2006
-
- Tang OS, Ho PC. Clinical applications of mifepristone. Gynecological Endocrinology 2006;22(12):655‐9. - PubMed
Tang 2007
-
- Tang OS, Gemzell‐Danielsson K, Ho PC. Misoprostol: pharmacokinetic profiles, effects on the uterus and side effects. International Journal of Gynecology & Obstetrics 2007;99:S160‐S167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

