Nucleotide Excision Repair and Transcription-coupled DNA Repair Abrogate the Impact of DNA Damage on Transcription
- PMID: 26559971
- PMCID: PMC4705403
- DOI: 10.1074/jbc.M115.685271
Nucleotide Excision Repair and Transcription-coupled DNA Repair Abrogate the Impact of DNA Damage on Transcription
Abstract
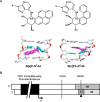
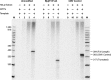
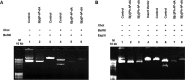
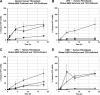
DNA adducts derived from carcinogenic polycyclic aromatic hydrocarbons like benzo[a]pyrene (B[a]P) and benzo[c]phenanthrene (B[c]Ph) impede replication and transcription, resulting in aberrant cell division and gene expression. Global nucleotide excision repair (NER) and transcription-coupled DNA repair (TCR) are among the DNA repair pathways that evolved to maintain genome integrity by removing DNA damage. The interplay between global NER and TCR in repairing the polycyclic aromatic hydrocarbon-derived DNA adducts (+)-trans-anti-B[a]P-N(6)-dA, which is subject to NER and blocks transcription in vitro, and (+)-trans-anti-B[c]Ph-N(6)-dA, which is a poor substrate for NER but also blocks transcription in vitro, was tested. The results show that both adducts inhibit transcription in human cells that lack both NER and TCR. The (+)-trans-anti-B[a]P-N(6)-dA lesion exhibited no detectable effect on transcription in cells proficient in NER but lacking TCR, indicating that NER can remove the lesion in the absence of TCR, which is consistent with in vitro data. In primary human cells lacking NER, (+)-trans-anti-B[a]P-N(6)-dA exhibited a deleterious effect on transcription that was less severe than in cells lacking both pathways, suggesting that TCR can repair the adduct but not as effectively as global NER. In contrast, (+)-trans-anti-B[c]Ph-N(6)-dA dramatically reduces transcript production in cells proficient in global NER but lacking TCR, indicating that TCR is necessary for the removal of this adduct, which is consistent with in vitro data showing that it is a poor substrate for NER. Hence, both global NER and TCR enhance the recovery of gene expression following DNA damage, and TCR plays an important role in removing DNA damage that is refractory to NER.
Keywords: DNA repair; RNA polymerase II; carcinogenesis; nucleotide excision repair; transcription.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



Similar articles
-
DNA adducts from a tumorigenic metabolite of benzo[a]pyrene block human RNA polymerase II elongation in a sequence- and stereochemistry-dependent manner.J Mol Biol. 2002 Aug 2;321(1):29-47. doi: 10.1016/s0022-2836(02)00593-4. J Mol Biol. 2002. PMID: 12139931
-
Base excision repair and nucleotide excision repair contribute to the removal of N-methylpurines from active genes.DNA Repair (Amst). 2002 Aug 6;1(8):683-96. doi: 10.1016/s1568-7864(02)00075-7. DNA Repair (Amst). 2002. PMID: 12509290
-
Differential removal of DNA adducts derived from anti-diol epoxides of dibenzo[a,l]pyrene and benzo[a]pyrene in human cells.Chem Res Toxicol. 2005 Apr;18(4):655-64. doi: 10.1021/tx0497090. Chem Res Toxicol. 2005. PMID: 15833025
-
Involvement of mismatch repair in transcription-coupled nucleotide excision repair.Hum Cell. 2005 Sep;18(3):103-15. doi: 10.1111/j.1749-0774.2005.tb00001.x. Hum Cell. 2005. PMID: 17022143 Review.
-
Nucleotide excision repair and its interplay with transcription.Toxicology. 2003 Nov 15;193(1-2):79-90. doi: 10.1016/j.tox.2003.06.001. Toxicology. 2003. PMID: 14599769 Review.
Cited by
-
Molecular basis for damage recognition and verification by XPC-RAD23B and TFIIH in nucleotide excision repair.DNA Repair (Amst). 2018 Nov;71:33-42. doi: 10.1016/j.dnarep.2018.08.005. Epub 2018 Aug 23. DNA Repair (Amst). 2018. PMID: 30174301 Free PMC article. Review.
-
Nucleotide excision repair as a targetable vulnerability in leukemia.Oncotarget. 2017 Dec 6;8(70):114420-114421. doi: 10.18632/oncotarget.22998. eCollection 2017 Dec 29. Oncotarget. 2017. PMID: 29383087 Free PMC article. No abstract available.
-
The torpedo effect in Bacillus subtilis: RNase J1 resolves stalled transcription complexes.EMBO J. 2020 Feb 3;39(3):e102500. doi: 10.15252/embj.2019102500. Epub 2019 Dec 16. EMBO J. 2020. PMID: 31840842 Free PMC article.
-
O6-methylguanine-induced transcriptional mutagenesis reduces p53 tumor-suppressor function.Proc Natl Acad Sci U S A. 2018 May 1;115(18):4731-4736. doi: 10.1073/pnas.1721764115. Epub 2018 Apr 17. Proc Natl Acad Sci U S A. 2018. PMID: 29666243 Free PMC article.
-
Transcriptional mutagenesis reduces splicing fidelity in mammalian cells.Nucleic Acids Res. 2017 Jun 20;45(11):6520-6529. doi: 10.1093/nar/gkx339. Nucleic Acids Res. 2017. PMID: 28460122 Free PMC article.
References
-
- Furgason J. M., and Bahassi el M. (2013) Targeting DNA repair mechanisms in cancer. Pharmacol. Ther. 137, 298–308 - PubMed
-
- Langie S. A., Koppen G., Desaulniers D., Al-Mulla F., Al-Temaimi R., Amedei A., Azqueta A., Bisson W. H., Brown D. G., Brunborg G., Charles A. K., Chen T., Colacci A., Darroudi F., Forte S., Gonzalez L., Hamid R. A., Knudsen L. E., Leyns L., Lopez de Cerain Salsamendi A., Memeo L., Mondello C., Mothersill C., Olsen A. K., Pavanello S., Raju J., Rojas E., Roy R., Ryan E. P., Ostrosky-Wegman P., Salem H. K., Scovassi A. I., Singh N., Vaccari M., Van Schooten F. J., Valverde M., Woodrick J., Zhang L., van Larebeke N., Kirsch-Volders M., and Collins A. R. (2015) Causes of genome instability: the effect of low dose chemical exposures in modern society. Carcinogenesis 36, S61-S88 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

