Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer
- PMID: 26560028
- PMCID: PMC4849281
- DOI: 10.1038/nature16064
Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer
Abstract
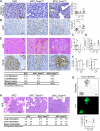
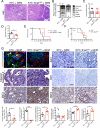
Diagnosis of pancreatic ductal adenocarcinoma (PDAC) is associated with a dismal prognosis despite current best therapies; therefore new treatment strategies are urgently required. Numerous studies have suggested that epithelial-to-mesenchymal transition (EMT) contributes to early-stage dissemination of cancer cells and is pivotal for invasion and metastasis of PDAC. EMT is associated with phenotypic conversion of epithelial cells into mesenchymal-like cells in cell culture conditions, although such defined mesenchymal conversion (with spindle-shaped morphology) of epithelial cells in vivo is rare, with quasi-mesenchymal phenotypes occasionally observed in the tumour (partial EMT). Most studies exploring the functional role of EMT in tumours have depended on cell-culture-induced loss-of-function and gain-of-function experiments involving EMT-inducing transcription factors such as Twist, Snail and Zeb1 (refs 2, 3, 7-10). Therefore, the functional contribution of EMT to invasion and metastasis remains unclear, and genetically engineered mouse models to address a causal connection are lacking. Here we functionally probe the role of EMT in PDAC by generating mouse models of PDAC with deletion of Snail or Twist, two key transcription factors responsible for EMT. EMT suppression in the primary tumour does not alter the emergence of invasive PDAC, systemic dissemination or metastasis. Suppression of EMT leads to an increase in cancer cell proliferation with enhanced expression of nucleoside transporters in tumours, contributing to enhanced sensitivity to gemcitabine treatment and increased overall survival of mice. Collectively, our study suggests that Snail- or Twist-induced EMT is not rate-limiting for invasion and metastasis, but highlights the importance of combining EMT inhibition with chemotherapy for the treatment of pancreatic cancer.
Figures








Comment in
-
Cell fate: Transition loses its invasive edge.Nature. 2015 Nov 26;527(7579):452-3. doi: 10.1038/nature16313. Epub 2015 Nov 11. Nature. 2015. PMID: 26560026 No abstract available.
References
-
- Hotz B, et al. Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:4769–4776. doi:10.1158/1078-0432.CCR-06-2926. - PubMed
-
- Taube JH, et al. Core epithelial-to-mesenchymal transition interactome gene- expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15449–15454. doi:10.1073/pnas.1004900107. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

