Bruch's membrane abnormalities in PRDM5-related brittle cornea syndrome
- PMID: 26560304
- PMCID: PMC4642625
- DOI: 10.1186/s13023-015-0360-4
Bruch's membrane abnormalities in PRDM5-related brittle cornea syndrome
Abstract
Background: Brittle cornea syndrome (BCS) is a rare, generalized connective tissue disorder associated with extreme corneal thinning and a high risk of corneal rupture. Recessive mutations in transcription factors ZNF469 and PRDM5 cause BCS. Both transcription factors are suggested to act on a common pathway regulating extracellular matrix genes, particularly fibrillar collagens. We identified bilateral myopic choroidal neovascularization as the presenting feature of BCS in a 26-year-old-woman carrying a novel PRDM5 mutation (p.Glu134*). We performed immunohistochemistry of anterior and posterior segment ocular tissues, as expression of PRDM5 in the eye has not been described, or the effects of PRDM5-associated disease on the retina, particularly the extracellular matrix composition of Bruch's membrane.
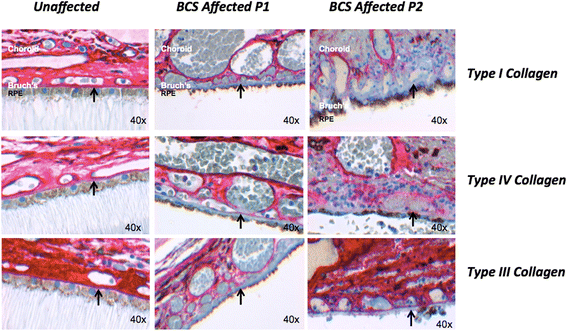
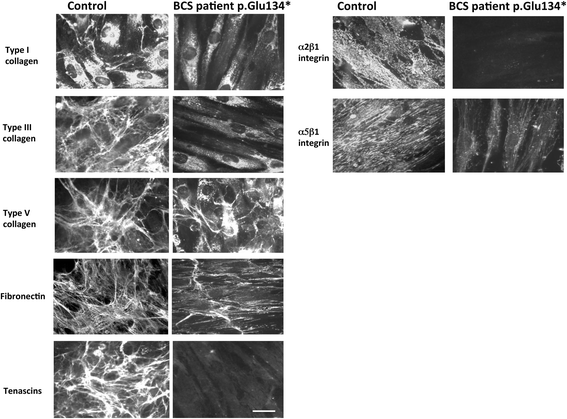
Methods: Immunohistochemistry using antibodies against PRDM5, collagens type I, III, and IV was performed on the eyes of two unaffected controls and two patients (both with Δ9-14 PRDM5). Expression of collagens, integrins, tenascin and fibronectin in skin fibroblasts of a BCS patient with a novel p.Glu134* PRDM5 mutation was assessed using immunofluorescence.
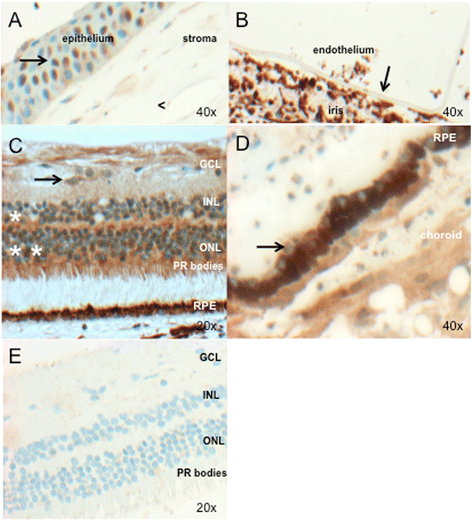
Results: PRDM5 is expressed in the corneal epithelium and retina. We observe reduced expression of major components of Bruch's membrane in the eyes of two BCS patients with a PRDM5 Δ9-14 mutation. Immunofluorescence performed on skin fibroblasts from a patient with p.Glu134* confirms the generalized nature of extracellular matrix abnormalities in BCS.
Conclusions: PDRM5-related disease is known to affect the cornea, skin and joints. Here we demonstrate, to the best of our knowledge for the first time, that PRDM5 localizes not only in the human cornea, but is also widely expressed in the retina. Our findings suggest that ECM abnormalities in PRDM5-associated disease are more widespread than previously reported.
Figures


References
-
- Rohrbach M, Spencer H, Porter LF, Burkitt-Wright EM, Bürer C, Janecke A, et al. ZNF469, frequently mutated in the Brittle Cornea Syndrome (BCS), is a single exon gene possibly regulating the expression of several extracellular matrix components. Mol Genet Metab. 2013;109(3):289–95. doi: 10.1016/j.ymgme.2013.04.014. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

