Adjuvant corticosteroids for reducing death in neonatal bacterial meningitis
- PMID: 26560739
- PMCID: PMC10542916
- DOI: 10.1002/14651858.CD010435.pub2
Adjuvant corticosteroids for reducing death in neonatal bacterial meningitis
Abstract
Background: Bacterial meningitis remains a significant cause of neonatal and childhood morbidity and mortality in many countries of the world, particularly in developing countries. In some instances, children recover but remain impaired as a result of neurological sequelae such as hearing loss, developmental delay and cognitive impairment.
Objectives: To assess the effectiveness and safety of adjunctive corticosteroids in reducing death and neurological sequelae in neonates with bacterial meningitis.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 7), MEDLINE via PubMed (1966 to July 2015), African Index Medicus (up to January 2015), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (up to July 2015), EMBASE (up to July 2015) and the metaRegister of Controlled Trials (mRCT) for ongoing trials.
Selection criteria: All randomised controlled trials (RCTs) or quasi-RCTs of adjunctive corticosteroids for treatment of neonates with bacterial meningitis.
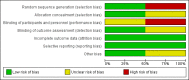
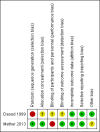
Data collection and analysis: Two review authors independently assessed and extracted data on methods, participants, interventions and outcomes (all-cause death until hospital discharge, presence of sensorineural deafness at one year and presence of neurological deficits or developmental delay at two years, adverse events). Risk ratio (RR), risk difference (RD) and number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) were calculated when appropriate. We assessed quality using the Cochrane risk of bias assessment tool and the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system.
Main results: We found two trials with 132 participants that met our inclusion criteria. One of the included trials was a quasi-randomised trial.Adjunctive corticosteroids reduced the risk of death (typical RR 0.46, 95% confidence interval (CI) 0.24 to 0.88; typical RD -0.19, 95% CI -0.33 to -0.04; NNTB = 6; two studies, 132 participants, very low-quality evidence) but did not have a significant effect on the number of infants with sensorineural deafness at two years (RR 1.80, 95% CI 0.18 to 18.21; RD 0.04, 95% CI -0.12 to 0.21; one study, 38 participants, low-quality evidence). In one trial, dexamethasone reduced the likelihood of hearing loss at four to 10 weeks post discharge (RR 0.41, 95% CI 0.17 to 0.98; RD -0.25, 95% CI -0.48 to -0.01; one study, 59 participants, low-quality evidence). Data reported on the other outcomes of interest were insufficient.
Authors' conclusions: Very low-quality data from two randomised controlled trials suggest that some reduction in death and hearing loss may result from use of adjunctive steroids alongside standard antibiotic therapy for treatment of patients with neonatal meningitis. Benefit is not yet seen with regards to reduction in neurological sequelae. Researchers who wish to clarify these findings must conduct more robustly designed trials with greater numbers of participants, evaluating more relevant outcomes and providing adequate follow-up.
Conflict of interest statement
None.
Figures







Update of
References
References to studies included in this review
Daoud 1999 {published data only}
-
- Daoud AS, Batieha A, Al‐Sheyyab M, Abueikteish F, Obeidat A, Mahafza T. Lack of effectiveness of dexamethasone in neonatal bacterial meningitis. European Journal of Pediatrics 1999;158(3):230‐3. [PUBMED: 10094445] - PubMed
Mathur 2013 {published data only}
-
- Mathur NB, Garg A, Mishra TK. Role of dexamethasone in neonatal meningitis: a randomized controlled trial. Indian Journal of Pediatrics 2013;80(2):102‐7. [PUBMED: 23054852] - PubMed
References to studies excluded from this review
Mathur 2015 {published data only}
-
- Mathur NB, Kharod P, Kumar S. Evaluation of duration of antibiotic therapy in neonatal bacterial meningitis: a randomized controlled trial. Journal of Tropical Pediatrics 2015;61(2):119‐25. [PUBMED: 25681965] - PubMed
Additional references
Airede 2008
-
- Airede KI, Adeyemi O, Ibrahim T. Neonatal bacterial meningitis and dexamethasone adjunctive usage in Nigeria. Nigerian Journal of Clinical Practice 2008;11(3):235‐45. [PUBMED: 19140361] - PubMed
Brouwer 2010
Brouwer 2013
Chang 2003
-
- Chang CJ, Chang WN, Huang LT, Huang SC, Chang YC, Hung PL, et al. Neonatal bacterial meningitis in southern Taiwan. Pediatric Neurology 2003;29(4):288‐94. [PUBMED: 14643389] - PubMed
Delouvois 1991
Furyk 2011
-
- Furyk JS, Swann O, Molyneux E. Systematic review: neonatal meningitis in the developing world. Tropical Medicine and International Health 2011;16(6):672‐9. [PUBMED: 21395927] - PubMed
Guyatt 2011
-
- Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles. Journal of Clinical Epidemiology 2011;64(4):380‐2. [PUBMED: 21185693] - PubMed
Harvey 1999
-
- Harvey D, Holt D, Bedford H. Bacterial meningitis in the newborn: a prospective study of mortality and morbidity. Seminars in Perinatology 1999;23(3):218‐25. [PUBMED: 10405191] - PubMed
Higgins 2002
-
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Laving 2003
-
- Laving AM, Musoke RN, Wasunna AO, Revathi G. Neonatal bacterial meningitis at the newborn unit of Kenyatta National Hospital. East African Medical Journal 2003;80(9):456‐62. [PUBMED: 14640166] - PubMed
McIntyre 1997
-
- McIntyre PB, Berkey CS, King SM, Schaad UB, Kilpi T, Kanra GY, et al. Dexamethasone as adjunctive therapy in bacterial meningitis. A meta‐analysis of randomized clinical trials since 1988. JAMA 1997;278(11):925‐31. [PUBMED: 9302246] - PubMed
Molyneux 2002
-
- Molyneux EM, Walsh AL, Forsyth H, Tembo M, Mwenechanya J, Kayira K, et al. Dexamethasone treatment in childhood bacterial meningitis in Malawi: a randomized controlled trial. Lancet 2002;360(9328):211‐8. [PUBMED: 12133656] - PubMed
Mustafa 1990
-
- Mustafa MM, Ramilo O, Saez Liorens X, Olsen KD, Magness RR, McCracken GH Jr. Cerebrospinal fluid prostaglandins, interleukins 1 beta, and tumor necrosis factor in bacterial meningitis. Clinical and laboratory correlations in placebo treated and dexamethasone treated patients. American Journal of Diseases of Children 1990;144(8):883‐7. [PUBMED: 2116086] - PubMed
Odio 1991
-
- Odio CM, Faingezicht I, Paris M, Nassar M, Baltodano A, Rogers J, et al. The beneficial effects of early dexamethasone administration in infants and children with bacterial meningitis. New England Journal of Medicine 1991;324(22):1525‐31. [PUBMED: 2027357] - PubMed
Rang 2003
-
- Rang HP, Dale MM, Ritter JM, Moore PK. The pituitary and adrenal cortex. In: Hunter I editor(s). Pharmacology. 5th Edition. Edinburgh: Churchill Livingstone, 2003.
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Stoll 1997
-
- Stoll B. The global impact of neonatal infection. Clinics in Perinatology 1997;24(1):1‐21. [PUBMED: 9099499] - PubMed
Wald 1995
-
- Wald ER, Kaplan SL, Mason EO Jr, Sabo D, Ross L, Arditi M, et al. Dexamethasone therapy for children with bacterial meningitis. Meningitis Study Group. Pediatrics 1995;95(1):21‐8. [PUBMED: 7770303] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

