The unfolded protein response: the dawn of a new field
- PMID: 26560836
- PMCID: PMC4754504
- DOI: 10.2183/pjab.91.469
The unfolded protein response: the dawn of a new field
Abstract
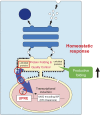
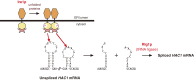
Originating from cancer research in mammalian cultured cells, the entirely new field of the unfolded protein response (UPR) was born in 1988. The UPR is a transcriptional induction program coupled with intracellular signaling from the endoplasmic reticulum (ER) to the nucleus to maintain the homeostasis of the ER, an organelle which controls the quality of proteins destined for the secretory pathway. Extremely competitive analyses using the budding yeast Saccharomyces cerevisiae revealed that although signaling from both the ER and cell surface is initiated by activation of a transmembrane protein kinase, the mechanism downstream of ER-resident Ire1p, a sensor molecule of the UPR, is unique. Thus, unconventional spliceosome-independent mRNA splicing is utilized to produce the highly active transcription factor Hac1p. This is the autobiographical story of how a young and not yet independent scientist competed with a very famous full professor in the early days of UPR research, which ultimately lead to their sharing Lasker Basic Medical Research Award in 2014.
Figures
References
-
- Gething M.J., Sambrook J. (1992) Protein folding in the cell. Nature 355, 33–45. - PubMed
-
- Helenius A., Marquardt T., Braakman I. (1992) The endoplasmic reticulum as a protein folding compartment. Trends Cell Biol. 2, 227–231. - PubMed
-
- Brodsky J.L., McCracken A.A. (1997) ER-associated and proteasome-mediated protein degradation: how two topologically restricted events came together. Trends Cell Biol. 7, 151–156. - PubMed
-
- Mori K. (2000) Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell 101, 451–454. - PubMed
-
- Stone K.R., Smith R.E., Joklik W.K. (1974) Changes in membrane polypeptides that occur when chick embryo fibroblasts and NRK cells are transformed with avian sarcoma viruses. Virology 58, 86–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

