Interventions for metabolic bone disease in children with chronic kidney disease
- PMID: 26561037
- PMCID: PMC7180137
- DOI: 10.1002/14651858.CD008327.pub2
Interventions for metabolic bone disease in children with chronic kidney disease
Abstract
Background: Bone disease is common in children with chronic kidney disease (CKD) and when untreated may result in bone deformities, bone pain, fractures and reduced growth rates. This is an update of a review first published in 2010.
Objectives: This review aimed to examine the benefits (improved growth rates, reduced risk of bone fractures and deformities, reduction in PTH levels) and harms (hypercalcaemia, blood vessel calcification, deterioration in kidney function) of interventions (including vitamin D preparations and phosphate binders) for the prevention and treatment of metabolic bone disease in children with CKD.
Search methods: We searched the Cochrane Kidney and Transplant Specialised Register to 8 September 2015 through contact with the Trial's Search Co-ordinator using search terms relevant for this review.
Selection criteria: We included randomised controlled trials (RCTs) comparing different interventions used to prevent or treat bone disease in children with CKD stages 2 to 5D.
Data collection and analysis: Data were assessed for study eligibility, risk of bias and extracted independently by two authors. Results were reported as risk ratios (RR) or risk differences (RD) with 95% confidence intervals (CI) for dichotomous outcomes. For continuous outcomes the mean difference (MD) or standardised mean difference (SMD) with 95% confidence intervals (CI) was used. Statistical analyses were performed using the random-effects model.
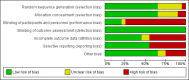
Main results: This review included 18 studies (576 children); three new studies were added for this update. Adequate sequence generation and allocation concealment were reported in 12 and 11 studies respectively. Only four studies reported blinding of children, investigators or outcome assessors. Nine studies were at low risk of attrition bias and 12 studies were at low risk of selective reporting bias.Eight different interventions were compared. Two studies compared intraperitoneal (IP) with oral calcitriol. PTH levels were significantly lower with IP compared with oral calcitriol (1 study: MD -501.00 pg/mL, 95% CI -721.54 to -280.46) but the number of children with abnormal bone histology did not differ between treatments. Three studies compared intermittent with daily oral calcitriol. The change in mean height SDS (1 study: MD 0.13, 95% CI -0.22 to 0.48) and the percentage fall in parathyroid hormone (PTH) levels at eight weeks (1 study: MD -5.50%, 95% CI -32.37 to 21.37) and 12 months (1 study: MD -6.00% 95% CI -25.27 to 13.27) did not differ between treatments.Four studies compared active vitamin D preparations (calcitriol, paricalcitol, 1α-hydroxyvitamin D) with placebo or no specific treatment. One study reported vitamin D preparations significantly reduced PTH levels (-55.00 pmol/L, 95% CI -83.03 to -26.97). There was no significant difference in hypercalcaemia risk with vitamin D preparations compared with placebo or no specific treatment (4 studies, 103 children: RD 0.08 mg/dL, 95% CI -0.08 to 0.24). However, there was heterogeneity (I(2) = 55%) with one study showing a significantly greater risk of hypercalcaemia with intravenous (IV) calcitriol administration. Two studies (97 children) compared calcitriol with other vitamin D preparations and both found no significant differences in growth between preparations.Two studies compared ergocalciferol in patients with CKD and vitamin D deficiency. Elevated PTH levels developed significantly later in ergocalciferol treated children (1 study: hazard ratio 0.30, 95% CI 0.09 to 0.93) though the number with elevated PTH levels did not differ between groups (1 study, 40 children: RR 0.33, 95% CI 0.11 to 1.05).Two studies compared calcium carbonate with aluminium hydroxide as phosphate binders. One study (17 children: MD -0.86 SDS, 95% CI -2.24 to 0.52) reported no significant difference in mean final height SDS between treatments. Three studies compared sevelamer with calcium-containing phosphate binders. There were no significant differences in the final calcium, phosphorus or PTH levels between binders. More episodes of hypercalcaemia occurred with calcium-containing binders. One study reported no significant differences between calcitriol and doxercalciferol in bone histology or biochemical parameters.
Authors' conclusions: Bone disease, assessed by changes in PTH levels, is improved by all vitamin D preparations. However, no consistent differences between routes of administration, frequencies of dosing or vitamin D preparations were demonstrated. Although fewer episodes of high calcium levels occurred with the non-calcium-containing phosphate binder, sevelamer, compared with calcium-containing binders, there were no differences in serum phosphorus and calcium overall and phosphorus values were reduced to similar extents. All studies were small with few data available on patient-centred outcomes (growth, bone deformities) and limited data on biochemical parameters or bone histology resulting in considerable imprecision of results thus limiting the applicability to the care of children with CKD.
Conflict of interest statement
Elisabeth Hodson: I was the lead author on a study published in 1985 (Hodson 1985), which was eligible for and included in this review. Data extraction was carried out independently by Dr Geary and myself and all data included in the review was agreed on by both authors.
Deirdre Hahn: none declared
Jonathan Craig: none declared
Figures








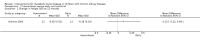















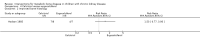





















Update of
-
Interventions for bone disease in children with chronic kidney disease.Cochrane Database Syst Rev. 2010 Jan 20;(1):CD008327. doi: 10.1002/14651858.CD008327. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2015 Nov 12;(11):CD008327. doi: 10.1002/14651858.CD008327.pub2. PMID: 20091666 Updated.
References
References to studies included in this review
Ardissino 2000 {published data only}
-
- Ardissino G, Schmitt CP, Testa S, Claris‐Appiani A, Mehls O. Calcitriol pulse therapy is not more effective than daily calcitriol therapy in controlling secondary hyperparathyroidism in children with chronic renal failure. European Study Group on Vitamin D in Children with Renal Failure. Pediatric Nephrology 2000;14(7):664‐68. [MEDLINE: ] - PubMed
-
- Ardissino G, Schmitt CP, Testa S, Claris‐Appiani A, Mehls O, European Study Group on Vitamin D in Children with (Chronic) Renal Failure. Growth and PTH in children treated with daily vs intermittent oral calcitriol for secondary hyperparathyroidism. Preliminary results [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):562A. [CENTRAL: CN‐00444220]
Eke 1983 {published data only}
-
- Eke FU, Winterborn MH. Effect of 1a‐hydroxycholecalciferol on glomerular filtration rate in children with moderate renal failure [abstract]. Kidney International 1982;22(5):581. [CENTRAL: CN‐00583351]
GFRD Study 1990 {published data only}
-
- Abitbol CL, Warady BA, Massie MD, Baluarte HJ, Fleischman LE, Geary DF, et al. Linear growth and anthropometric and nutritional measurements in children with mild to moderate renal insufficiency: a report of the Growth Failure in Children with Renal Diseases Study. Journal of Pediatrics 1990;116(2):S46‐54. [MEDLINE: ] - PubMed
-
- Anonymous. Growth failure in children with renal diseases. A clinical trial in pediatric nephrology: Growth Failure in Renal Diseases (GFRD) Study. Journal of Pediatrics 1990;116(2):S1‐62. [MEDLINE: ] - PubMed
-
- Boyle RM, Chinchilli VM, Shasky DA. Masking of physicians in the Growth Failure in Children with Renal Diseases Clinical Trial. Pediatric Nephrology 1993;7(2):204‐6. [MEDLINE: ] - PubMed
-
- Boyle RM, Fortner CA, Chinchilli VM, Chan JC. Data coordination and management in the Growth Failure in Children with Renal Diseases Study. Journal of Pediatrics 1990;116(2):S28‐31. [MEDLINE: ] - PubMed
-
- Brouhard BH, Chan JC, Carter WH Jr. Final report of the Growth Failure in Children with Renal Diseases Study. Journal of Pediatrics 1996;129(2):S1‐3. [MEDLINE: ] - PubMed
Greenbaum 2005 {published data only}
-
- Greenbaum LA, Grenda R, Qiu P, Restaino I, Wojtak A, Paredes A, et al. Intravenous calcitriol for treatment of hyperparathyroidism in children on hemodialysis. Pediatric Nephrology 2005;20(5):622‐30. [MEDLINE: ] - PubMed
-
- Salusky IB, Greenbaum L, Grenda R, Qiu P, Langman CB. IV calcitriol safely and effectively reduces iPTH levels in pediatric patients [abstract no: A4031]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):771A‐2AS. [CENTRAL: CN‐00765519]
Greenbaum 2007 {published data only}
-
- Greenbaum LA, Benador N, Goldstein SL, Paredes A, Melnick JZ, Mattingly S, et al. Intravenous paricalcitol for treatment of secondary hyperparathyroidism in children on hemodialysis. American Journal of Kidney Diseases 2007;49(6):814‐23. [MEDLINE: ] - PubMed
-
- Kommala DR, Benador N, Goldstein S, Pardes A, Mattingly S, Amdahl M, et al. Paricalcitol (Zemplar®) injection for the treatment of secondary hyperparathyroidism in pediatric hemodialysis patients [abstract]. Journal of the American Society of Nephrology 2003;14(Nov):199A. [CENTRAL: CN‐00583154]
Gulati 2010 {published data only}
-
- Gulati A, Sridhar V, Bose T, Hari P, Bagga A. Short‐term efficacy of sevelamer versus calcium acetate in patients with chronic kidney disease stage 3‐4. International Urology & Nephrology 2010;42(4):1055‐62. [MEDLINE: ] - PubMed
Hodson 1985 {published data only}
-
- Hodson EM, Evans RA, Dunstan CR, Hills E, Wong SY, Rosenberg AR, et al. Treatment of childhood renal osteodystrophy with calcitriol or ergocalciferol. Clinical Nephrology 1985;24(4):192‐200. [MEDLINE: ] - PubMed
Jones 1994 {published data only}
-
- Jones CL, Vieth R, Spino M, Ledermann S, Kooh SW, Balfe J, et al. Comparisons between oral and intraperitoneal 1,25‐dihydroxyvitamin D3 therapy in children treated with peritoneal dialysis. Clinical Nephrology 1994;42(1):44‐9. [MEDLINE: ] - PubMed
Klaus 1995 {published data only}
-
- Klaus G, Hinderer J, Lingens B, Keuth B, Querfield U, Mehls O. Comparison of intermittent and continuous (daily) oral calcitriol for treatment of renal hyperparathyroidism in children on dialysis [abstract]. Pediatric Nephrology 1995;9(6):C75. [CENTRAL: CN‐00550467]
Mak 1985 {published data only}
-
- Mak RH, Turner C, Thompson T, Powell H, Haycock GB, Chantler C. Suppression of secondary hyperparathyroidism in children with chronic renal failure by high dose phosphate binders: calcium carbonate versus aluminium hydroxide. British Medical Journal Clinical Research Ed 1985;291(6496):623‐7. [MEDLINE: ] - PMC - PubMed
Pieper 2006 {published data only}
-
- Pieper AK, Haffner D, Hoppe B, Dittrich K, Offner G, Bonzel KE, et al. A randomized crossover trial comparing sevelamer with calcium acetate in children with CKD. American Journal of Kidney Diseases 2006;47(4):625‐35. [MEDLINE: ] - PubMed
-
- Pieper AK, Haffner D, Muller‐Honger R, Hoppe B, Plank C, Offner G, et al. Sevelamer vs. calcium acetate in children with chronic renal failure [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):681‐2. [CENTRAL: CN‐00447215]
Rianthavorn 2013 {published data only}
-
- Rianthavorn P, Boonyapapong P. Ergocalciferol decreases erythropoietin resistance in children with chronic kidney disease stage 5. Pediatric Nephrology 2013;28(8):1261‐6. [MEDLINE: ] - PubMed
Salusky 1991 {published data only}
-
- Goodman WG, Coburn JW, Foley J, Salusky IB. Prospective assessment of aluminum retention in dialyzed patients given calcium carbonate or aluminum hydroxide [abstract]. Kidney International 1990;37(1):328. [CENTRAL: CN‐00626083]
-
- Salusky IB, Coburn JW, Foley J, Slatopolsky E, Goodman WG. Prospective trial of calcium carbonate versus aluminum hydroxide with oral calcitriol therapy in renal osteodystrophy [abstract]. Kidney International 1990;37(1):451.
-
- Salusky IB, Foley J, Goodman WG. Calcium carbonate versus aluminum hydroxide with oral calcitriol therapy in pediatric renal osteodystrophy. [abstract]. 11th International Congress of Nephrology; 1990 Jul 15‐20; Tokyo, Japan. 1990:416A.
-
- Salusky IB, Foley J, Nelson P, Goodman WG. Aluminum accumulation during treatment with aluminum hydroxide and dialysis in children and young adults with chronic renal disease. New England Journal of Medicine 1991;324(8):527‐31. [MEDLINE: ] - PubMed
Salusky 1998 {published data only}
-
- Goodman WG, Belin T, Gales B, Juppner H, Sgre GV, Salusky IB. Skeletal response to intermittent oral or intraperitoneal calcitriol in patients with secondary hyperparathyroidism [abstract]. Journal of the American Society of Nephrology 1994;5(3):850. [CENTRAL: CN‐00626086]
-
- Goodman WG, Ramirez JA, Gales B, Segre GV, Salusky IB. Skeletal response to one year of intermittent calcitriol therapy in dialyzed children [abstract no: 23P]. Journal of the American Society of Nephrology 1992;3(3):672. [CENTRAL: CN‐00460833]
-
- Greenbaum LA, Goodman WG, Holloway M, Chon Y, Gales B, Salusky IB. A prospective evaluation of the peritonitis rates between oral & intraperitoneal calcitriol [abstract]. Journal of the American Society of Nephrology 1995;6(3):531. [CENTRAL: CN‐00484167]
-
- Holloway MS, Ramirez JA, Goodman WG, Salusky IB. Peritonitis rates in children on CCPD treated with intermittent calcitriol therapy: oral vs intraperitoneal [abstract]. Journal of the American Society of Nephrology 1992;3(3):412. [CENTRAL: CN‐00460953]
-
- Kuizon BD, Goodman WG, Juppner H, Boechat I, Nelson P, Gales B, et al. Diminished linear growth during intermittent calcitriol therapy in children undergoing CCPD. Kidney International 1998;53(1):205‐11. [MEDLINE: ] - PubMed
Salusky 2005 {published data only}
-
- Hernandez J, Wesseling K, Jueppner H, Goodman WG, Gales B, Elashoff R, et al. Skeletal response to phosphate binders and intermittent oral vitamin D in dialyzed children with secondary hyperparathyroidism [abstract no: F‐FC044]. Journal of the American Society of Nephrology 2005;16:47A.
-
- Pereira RC, Hernandez JD, Wesseling K, Gales B, Jueppner HW, Salusky IB. Differential effect of doxercalciferol and calcitriol on skeletal lesions of secondary hyperparathyroidism [abstract no: TH‐PO133]. Journal of the American Society of Nephrology 2006;17(Abstracts):134A. [CENTRAL: CN‐00740590]
-
- Salusky I, Jueppner H, Gales B, Elashoff R, Goodman WG. Skeletal response to the treatment of secondary hyperparathyroidism: a comparison between calcitriol and 1‐Alpha‐D2 [abstract no: F‐PO643]. Journal of the American Society of Nephrology 2003;14:202A.
-
- Salusky IB, Goodman WG, Elashoff R, Gales B, Shaney S, Jueppner H. Prospective comparison of calcium‐based binder versus sevelamer HCL on the control of the skeletal lesions of secondary hyperparathyroidism [abstract no: F‐PO989]. Journal of the American Society of Nephrology 2004;15:281A.
-
- Salusky IB, Goodman WG, Sahney S, Gales B, Perilloux A, Wang HJ, et al. Sevelamer controls parathyroid hormone‐induced bone disease as efficiently as calcium carbonate without increasing serum calcium levels during therapy with active vitamin D sterols. Journal of the American Society of Nephrology 2005;16(8):2501‐08. [MEDLINE: ] - PubMed
Salusky 2005a {published data only}
-
- Pereira RC, Hernandez JD, Wesseling K, Gales B, Jueppner HW, Salusky IB. Differential effect of doxercalciferol and calcitriol on skeletal lesions of secondary hyperparathyroidism [abstract no: TH‐PO133]. Journal of the American Society of Nephrology 2006;17(Abstracts):134A. [CENTRAL: CN‐00740590]
-
- Salusky I, Jueppner H, Gales B, Elashoff R, Goodman WG. Skeletal response to the treatment of secondary hyperparathyroidism: a comparison between calcitriol and 1‐Alpha‐D2 [abstract no: F‐PO643]. Journal of the American Society of Nephrology 2003;14:202A.
-
- Salusky IB, Wesseling K, Hernandez J, Lavigne JR, Zahranik RJ, Gales B, et al. Comparison between first and second generation PTH assays as predictors of bone turnover during treatment of secondary hyperparathyroidism in dialyzed children [abstract no: SA‐PO833]. Journal of the American Society of Nephrology 2005;16:739A.
-
- Wesseling‐Perry K, Jueppner H, Goodman WG, Gales B, Elasoff R, Wang H, et al. Similar predictive value of bone turnover by first and second generation PTH assays during treatment of secondary hyperparthyroidism in dialyzed children [abstract no: F‐PO980]. Journal of the American Society of Nephrology 2004;15(Oct):279A.
Salusky 2005b {published and unpublished data}
-
- Pereira RC, Hernandez JD, Wesseling K, Gales B, Jueppner HW, Salusky IB. Differential effect of doxercalciferol and calcitriol on skeletal lesions of secondary hyperparathyroidism [abstract no: TH‐PO133]. Journal of the American Society of Nephrology 2006;17(Abstracts):134A. [CENTRAL: CN‐00740590]
-
- Salusky I, Jueppner H, Gales B, Elashoff R, Goodman WG. Skeletal response to the treatment of secondary hyperparathyroidism: a comparison between calcitriol and 1‐Alpha‐D2 [abstract no: F‐PO643]. Journal of the American Society of Nephrology 2003;14:202A.
-
- Salusky IB, Wesseling K, Hernandez J, Lavigne JR, Zahranik RJ, Gales B, et al. Comparison between first and second generation PTH assays as predictors of bone turnover during treatment of secondary hyperparathyroidism in dialyzed children [abstract no: SA‐PO833]. Journal of the American Society of Nephrology 2005;16:739A.
-
- Wesseling‐Perry K, Jueppner H, Goodman WG, Gales B, Elasoff R, Wang H, et al. Similar predictive value of bone turnover by first and second generation PTH assays during treatment of secondary hyperparthyroidism in dialyzed children [abstract no: F‐PO980]. Journal of the American Society of Nephrology 2004;15(Oct):279A.
Schmitt 2003 {published data only}
-
- Schmitt CP, Ardissino G, Testa S, Claris‐Appiani A, Mehls O. Growth in children with chronic renal failure on intermittent versus daily calcitriol. Pediatric Nephrology 2003;18(5):440‐4. [MEDLINE: ] - PubMed
Shroff 2012 {published data only}
-
- Shroff R, Wan M, Gullett A, Lederman S, Shute R, Knott C, et al. Ergocalciferol supplementation in children with CKD delays the onset of secondary hyperparathyroidism: a randomized trial. Clinical Journal of The American Society of Nephrology: CJASN 2012;7(2):216‐23. [MEDLINE: ] - PubMed
-
- Shroff R, Wan M, Gullett A, Ledermann S, Shute R, Knott C, et al. Ergocalciferol supplementation in children with CKD delays the onset of secondary hyperparathyroidism‐a randomised, double‐blinded placebo controlled study [abstract no: PS1‐THU‐506]. Pediatric Nephrology 2011;26(9):1634. - PubMed
Watson 1988 {published data only}
-
- Watson AR, Kooh SW, Tam C, Reilly B, Farine M, Balfe JW. The prevention of renal osteodystrophy (ROD) in children under going CAPD: a prospective randomised trial using 1 a hydroxycholecalciferol (1a) [abstract]. Pediatric Nephrology 1987;1(1):C38. [CENTRAL: CN‐00448294]
-
- Watson AR, Kooh SW, Tam CS, Reilly BJ, Balfe JW, Vieth R. Renal osteodystrophy in children on CAPD: a prospective trial of 1‐alpha‐hydroxycholecalciferol therapy. Child Nephrology & Urology 1988;9(4):220‐7. [MEDLINE: ] - PubMed
References to studies excluded from this review
Ardissino 2000a {published data only}
-
- Ardissino G, Schmitt CP, Bianchi ML, Dacco V, Claris‐Appiani A, Mehls O. No difference in intestinal strontium absorption after oral or IV calcitriol in children with secondary hyperparathyroidism. The European Study Group on Vitamin D in Children with Renal Failure. Kidney International 2000;58(3):981‐8. [MEDLINE: ] - PubMed
-
- Ardissino G, Schmitt CP, Claris‐Appiani A, Bianchi ML, Dacco V, Mehls O, et al. Equal intestinal calcium absorption and greater PTH suppression with oral vs IV calcitriol bolus in children with secondary hyperparathyroidism [abstract]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):571A. [CENTRAL: CN‐00444219]
-
- Ardissino GL, Schmitt CP, Dacco V, Bianchi ML, Claris‐Appiani A, Mehls O, et al. Oral and IV calcitriol pulse are equally effective on control of serum PTH and in stimulating intestinal calcium absorption [abstract no: A2821]. Journal of the American Society of Nephrology 1996;7(9):1810. [CENTRAL: CN‐00615905]
-
- Schmitt C, Ardissino G, Bianchi M, Mehls O, European Study Group on Vit D in children with CRF. Higher intestinal calcium absorption with iv calcitriol compared to oral in children with 2º hyperparathyroidism (HPT) [abstract]. Nephrology Dialysis Transplantation 1997;12(9):A115. [CENTRAL: CN‐00677748]
Bettinelli 1986 {published data only}
-
- Bettinelli A, Bianchi ML, Aimini E, Ortolani S, Soldati L, Edefonti A. Effects of 1,25‐dihydroxyvitamin‐D3 treatment on mineral balance in children with end stage renal disease undergoing chronic hemofiltration. Pediatric Research 1986;20(1):5‐8. [MEDLINE: ] - PubMed
Choudhary 2014 {published data only}
-
- Choudhary S, Agarwal I, Seshadri MS. Calcium and vitamin D for osteoprotection in children with new‐onset nephrotic syndrome treated with steroids: a prospective, randomized, controlled, interventional study. Pediatric Nephrology 2014;29(6):1025‐32. [MEDLINE: ] - PubMed
El Husseini 2004 {published data only}
-
- Husseini A, El‐Agroudy A, El‐Sayed M, Sobh M, Ghoneim M. Treatment of bone loss in renal transplant children and adolescents [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):544. [CENTRAL: CN‐00445216]
-
- Husseini A, Elagroudy A, Elsayed M, Sobh M, Ghoneim M. Treatment of bone loss with vitamin D in live related kidney transplant children and adolescents: a prospective randomized study. 41st Congress of ERA‐EDTA; 2004 May 15‐18; Lisbon (Portugal). 2004:93. [CENTRAL: CN‐00509176]
-
- Husseini AA, Agroudy AE, Sayed MF, Sobh MA, Ghoneim MA. Treatment of osteopenia and osteoporosis in renal transplant children and adolescents. Pediatric Transplantation 2004;8(4):357‐61. [MEDLINE: ] - PubMed
-
- El‐Husseini AA, El‐Agroudy AE, El‐Sayed M, Sobh MA, Ghoneim MA. A prospective randomized study for the treatment of bone loss with vitamin D during kidney transplantation in children and adolescents. American Journal of Transplantation 2004;4(12):2052‐7. [MEDLINE: ] - PubMed
Ferraris 2000 {published data only}
-
- Ferraris JR, Pasqualini T, Alonso G, Legal S, Sorroche P, Galich AM, et al. Effects of deflazacort vs. methylprednisone: a randomized study in kidney transplant patients. Pediatric Nephrology 2007;22(5):734‐41. [MEDLINE: ] - PubMed
-
- Ferraris JR, Pasqualini T, Legal S, Sorroche P, Galich AM, Pennisi P, et al. Effect of deflazacort versus methylprednisone on growth, body composition, lipid profile, and bone mass after renal transplantation. The Deflazacort Study Group. Pediatric Nephrology 2000;14(7):682‐8. [MEDLINE: ] - PubMed
Kim 2006b {published data only}
-
- Cho B, Kim S. Pamidronate therapy for preventing steroid‐induced bone loss in children receiving corticosteroid therapy [abstract no: F‐PO346]. Journal of the American Society of Nephrology 2004;15(Oct):142A. [CENTRAL: CN‐00583366]
-
- Kim SD, Cho BS. Pamidronate therapy for preventing steroid‐induced osteoporosis in children with nephropathy. Nephron 2006;102(3‐4):c81‐7. [MEDLINE: ] - PubMed
Witmer 1976 {published data only}
-
- Witmer G, Margolis A, Fontaine O, Fritsch J, Lenoir G, Broyer M, et al. Effects of 25‐ hydroxycholecalciferol on bone lesions of children with terminal renal failure. Kidney International 1976;10(5):395‐408. [MEDLINE: ] - PubMed
Additional references
Avila‐Diaz 2006
-
- Avila‐Diaz M, Matos M, Garcia‐Lopez E, Prado MD, Castro‐Vazquez F, Ventura MD, et al. Serum markers of low‐turnover bone disease in Mexican children with chronic kidney disease undergoing dialysis. Peritoneal Dialysis International 2006;26(1):78‐84. [MEDLINE: ] - PubMed
Bacchetta 2011
-
- Bacchetta J, Boutroy S, Vilayphiou N, Ranchin B, Fouque‐Aubert A, Basmaison O, et al. Bone assessment in children with chronic kidney disease: data from two new bone imaging techniques in a single‐center pilot study. Pediatric Nephrology 2011;26(4):587‐95. [MEDLINE: ] - PubMed
Bakkaloglu 2010
Bakkaloglu 2011
-
- Bakkaloglu SA, Borzych D, Ha IL, Serdaroglu E, Buscher R, Salas P, et al. Cardiac geometry in children receiving chronic peritoneal dialysis: findings from the International Pediatric Peritoneal Dialysis Network (IPPN) registry. Clinical Journal of The American Society of Nephrology: CJASN 2011;6(8):1926‐33. [MEDLINE: ] - PMC - PubMed
Blaszak 2005
-
- Blaszak RT, Mitsnefes MM, Ilyas M, Salman SD, Belcher SM, Brady DR. Hyperphosphatemia in children receiving peritoneal dialysis‐‐an educational program. Pediatric Nephrology 2005;20(7):967‐71. [MEDLINE: ] - PubMed
Borzych 2010
-
- Borzych D, Rees L, Ha IS, Chua A, Valles PG, Lipka M, et al. The bone and mineral disorder of children undergoing chronic peritoneal dialysis. Kidney International 2010;78(12):1295‐304. [MEDLINE: ] - PubMed
Goodman 2000
-
- Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, et al. Coronary‐artery calcification in young adults with end‐stage renal disease who are undergoing dialysis. New England Journal of Medicine 2000;342(20):1478‐83. [MEDLINE: ] - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hodson 1982
-
- Hodson EM, Evans RA, Dunstan CR, Hills EE, Shaw PF. Quantitative bone histology in children with chronic renal failure. Kidney International 1982;21(6):833‐9. [MEDLINE: ] - PubMed
Hodson 2012
Kandula 2011
-
- Kandula P, Dobre M, Schold JD, Schreiber MJ Jr, Mehrotra R, Naveethan SD. Vitamin D supplementation in chronic kidney disease: a systematic review and meta‐analysis of observational studies and randomized controlled trials. Clinical Journal of The American Society of Nephrology: CJASN 2011;6(1):50‐62. [MEDLINE: ] - PMC - PubMed
KDOQI 2005
-
- National Kidney Foundation. KDOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Children With Chronic Kidney Disease. www2.kidney.org/professionals/KDOQI/guidelines_pedbone/ (accessed 7 October 2015).
KDOQI 2009
-
- KDOQI Work Group. KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 Update. American Journal of Kidney Diseases 2009;53(3 Suppl 2):S1‐S123. [MEDLINE: ] - PubMed
Klaus 2006
Moe 2006
-
- Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, et al. Definition, evaluation and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney International 2006;69(11):1945‐53. [MEDLINE: ] - PubMed
Navaneethan 2011
NCT01277510
-
- NCT01277510. A randomized, double‐blind, placebo controlled study to assess the efficacy and safety of cinacalcet HCl in pediatric subjects with chronic kidney disease and secondary hyperparathyroidism receiving dialysis. clinicaltrials.gov/ct2/show/NCT01277510 (accessed 29 October 2015).
Palmer 2009a
Palmer 2009b
Sanchez 2008
-
- Sanchez CP. Mineral metabolism and bone abnormalities in children with chronic renal failure. Reviews in Endocrine & Metabolic Disorders 2008;9(2):131‐7. [MEDLINE: ] - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [MEDLINE: ] - PubMed
Seikaly 2003
-
- Seikaly MG, Ho PL, Emmett L, Fine RN, Tejani A. Chronic renal insufficiency in children: the 2001 Annual Report of the NAPRTCS. Pediatric Nephrology 2003;18(8):796‐804. [MEDLINE: ] - PubMed
Tonelli 2007
-
- Tonelli M, Wiebe N, Culleton B, Lee H, Klarenbach S, Shrive F, et al. Systematic review of the clinical efficacy and safety of sevelamer in dialysis patients. Nephrology Dialysis Transplantation 2007;22(10):2856‐66. [MEDLINE: ] - PubMed
Wesseling‐Perry 2012
Wesseling‐Perry 2013
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

