Animal models to study acute and chronic intestinal inflammation in mammals
- PMID: 26561503
- PMCID: PMC4641401
- DOI: 10.1186/s13099-015-0076-y
Animal models to study acute and chronic intestinal inflammation in mammals
Abstract
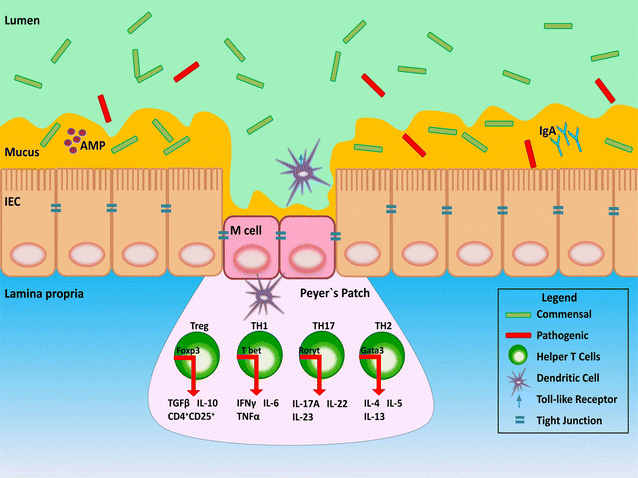
Acute and chronic inflammatory diseases of the intestine impart a significant and negative impact on the health and well-being of human and non-human mammalian animals. Understanding the underlying mechanisms of inflammatory disease is mandatory to develop effective treatment and prevention strategies. As inflammatory disease etiologies are multifactorial, the use of appropriate animal models and associated metrics of disease are essential. In this regard, animal models used alone or in combination to study acute and chronic inflammatory disease of the mammalian intestine paired with commonly used inflammation-inducing agents are reviewed. This includes both chemical and biological incitants of inflammation, and both non-mammalian (i.e. nematodes, insects, and fish) and mammalian (i.e. rodents, rabbits, pigs, ruminants, dogs, and non-human primates) models of intestinal inflammation including germ-free, gnotobiotic, as well as surgical, and genetically modified animals. Importantly, chemical and biological incitants induce inflammation via a multitude of mechanisms, and intestinal inflammation and injury can vary greatly according to the incitant and animal model used, allowing studies to ascertain both long-term and short-term effects of inflammation. Thus, researchers and clinicians should be aware of the relative strengths and limitations of the various animal models used to study acute and chronic inflammatory diseases of the mammalian intestine, and the scope and relevance of outcomes achievable based on this knowledge. The ability to induce inflammation to mimic common human diseases is an important factor of a successful animal model, however other mechanisms of disease such as the amount of infective agent to induce disease, invasion mechanisms, and the effect various physiologic changes can have on inducing damage are also important features. In many cases, the use of multiple animal models in combination with both chemical and biological incitants is necessary to answer the specific question being addressed regarding intestinal disease. Some incitants can induce acute responses in certain animal models while others can be used to induce chronic responses; this review aims to illustrate the strengths and weaknesses in each animal model and to guide the choice of an appropriate acute or chronic incitant to facilitate intestinal disease.
Keywords: Acute; Animal models; Biological; Chemical; Chronic; Incitants; Inflammation; Intestine.
Figures


References
-
- Akdis M. Healthy immune response to allergens: T regulatory cells and more. Curr Opin Immunol. 2006;18(6):738–744. - PubMed
-
- Shinoda M, Hatano S, Kawakubo H, Kakefuda T, Omori T, Ishii S. Adult cecoanal intussusception caused by cecum cancer: report of a case. Surg Today. 2007;37(9):802–805. - PubMed
-
- Dundas SAC, Dutton J, Skipworth P. Reliability of rectal biopsy in distinguishing between chronic inflammatory bowel disease and acute self-limiting colitis. Histopathology. 1997;31(1):60–66. - PubMed
-
- Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults (update): american college of gastroenterology, practice parameters committee. Am J Gastroenterol. 2004;99(7):1371–1385. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

