SNPase-ARMS qPCR: Ultrasensitive Mutation-Based Detection of Cell-Free Tumor DNA in Melanoma Patients
- PMID: 26562020
- PMCID: PMC4642939
- DOI: 10.1371/journal.pone.0142273
SNPase-ARMS qPCR: Ultrasensitive Mutation-Based Detection of Cell-Free Tumor DNA in Melanoma Patients
Abstract
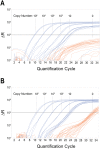
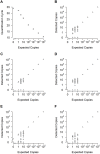
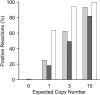
Cell-free circulating tumor DNA in the plasma of cancer patients has become a common point of interest as indicator of therapy options and treatment response in clinical cancer research. Especially patient- and tumor-specific single nucleotide variants that accurately distinguish tumor DNA from wild type DNA are promising targets. The reliable detection and quantification of these single-base DNA variants is technically challenging. Currently, a variety of techniques is applied, with no apparent "gold standard". Here we present a novel qPCR protocol that meets the conditions of extreme sensitivity and specificity that are required for detection and quantification of tumor DNA. By consecutive application of two polymerases, one of them designed for extreme base-specificity, the method reaches unprecedented sensitivity and specificity. Three qPCR assays were tested with spike-in experiments, specific for point mutations BRAF V600E, PTEN T167A and NRAS Q61L of melanoma cell lines. It was possible to detect down to one copy of tumor DNA per reaction (Poisson distribution), at a background of up to 200 000 wild type DNAs. To prove its clinical applicability, the method was successfully tested on a small cohort of BRAF V600E positive melanoma patients.
Conflict of interest statement
Figures



Similar articles
-
BRAF(V600E) and NRAS(Q61L/Q61R) mutation analysis in metastatic melanoma using immunohistochemistry: a study of 754 cases highlighting potential pitfalls and guidelines for interpretation and reporting.Histopathology. 2016 Oct;69(4):680-6. doi: 10.1111/his.12992. Epub 2016 Jun 15. Histopathology. 2016. PMID: 27151331
-
NRAS (Q61R), BRAF (V600E) immunohistochemistry: a concomitant tool for mutation screening in melanomas.Diagn Pathol. 2015 Jul 25;10:121. doi: 10.1186/s13000-015-0359-0. Diagn Pathol. 2015. PMID: 26204954 Free PMC article.
-
KIT, NRAS, BRAF and PTEN mutations in a sample of Swedish patients with acral lentiginous melanoma.J Dermatol Sci. 2013 Dec;72(3):284-9. doi: 10.1016/j.jdermsci.2013.07.013. Epub 2013 Aug 8. J Dermatol Sci. 2013. PMID: 23993026
-
Prenylation inhibition-induced cell death in melanoma: reduced sensitivity in BRAF mutant/PTEN wild-type melanoma cells.PLoS One. 2015 Feb 3;10(2):e0117021. doi: 10.1371/journal.pone.0117021. eCollection 2015. PLoS One. 2015. PMID: 25646931 Free PMC article.
-
Plasma Circulating Tumor DNA Levels for the Monitoring of Melanoma Patients: Landscape of Available Technologies and Clinical Applications.Biomed Res Int. 2017;2017:5986129. doi: 10.1155/2017/5986129. Epub 2017 Apr 6. Biomed Res Int. 2017. PMID: 28484715 Free PMC article. Review.
Cited by
-
Using cfDNA and ctDNA as Oncologic Markers: A Path to Clinical Validation.Int J Mol Sci. 2023 Aug 25;24(17):13219. doi: 10.3390/ijms241713219. Int J Mol Sci. 2023. PMID: 37686024 Free PMC article. Review.
-
DNA terminal structure-mediated enzymatic reaction for ultra-sensitive discrimination of single nucleotide variations in circulating cell-free DNA.Nucleic Acids Res. 2018 Feb 28;46(4):e24. doi: 10.1093/nar/gkx1218. Nucleic Acids Res. 2018. PMID: 29190359 Free PMC article.
-
Noninversion Variants in Sporadic Hemophilia A Originate Mostly from Females.Int J Mol Sci. 2025 Jan 22;26(3):891. doi: 10.3390/ijms26030891. Int J Mol Sci. 2025. PMID: 39940661 Free PMC article.
-
Liquid Biopsies for Cancer: Coming to a Patient near You.J Clin Med. 2017 Jan 4;6(1):3. doi: 10.3390/jcm6010003. J Clin Med. 2017. PMID: 28054963 Free PMC article. Review.
-
Translational Application of Circulating DNA in Oncology: Review of the Last Decades Achievements.Cells. 2019 Oct 14;8(10):1251. doi: 10.3390/cells8101251. Cells. 2019. PMID: 31615102 Free PMC article. Review.
References
-
- Haber DA, Velculescu VE. Blood-based analyses of cancer: circulating tumor cells and circulating tumor DNA. Cancer Discov. 2014;4(6):650–61. 10.1158/2159-8290.CD-13-1014 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

