Cascade of Care for Hepatitis C Virus Infection Within the US Veterans Health Administration
- PMID: 26562129
- PMCID: PMC4815568
- DOI: 10.2105/AJPH.2015.302927
Cascade of Care for Hepatitis C Virus Infection Within the US Veterans Health Administration
Abstract
Objectives: We measured the quality of HCV care using a cascade of HCV care model.
Methods: We estimated the number of patients diagnosed with chronic HCV, linked to HCV care, treated with HCV antivirals, and having achieved a sustained virologic response (SVR) in the electronic medical record data from the Veterans Health Administration's Corporate Data Warehouse and the HCV Clinical Case Registry in 2013.
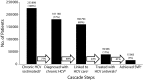
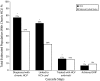
Results: Of the estimated 233,898 patients with chronic HCV, 77% (181,168) were diagnosed, 69% (160,794) were linked to HCV care, 17% (39,388) were treated with HCV antivirals, and 7% (15,983) had achieved SVR.
Conclusions: This Cascade of HCV Care provides a clinically relevant model to measure the quality of HCV care within a health care system and to compare HCV care across health systems.
Figures
References
-
- Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1992 through 2002. Ann Intern Med. 2006;144(10):705–714. - PubMed
-
- Backus LI, Belperio PS, Loomis TP, Yip GH, Mole LA. Hepatitis C virus screening and prevalence among US veterans in Department of Veterans Affairs care. JAMA Intern Med. 2013;173(16):1549–1552. - PubMed
-
- Fried MW, Shiffman ML, Reddy KR et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975–982. - PubMed
-
- Manns MP, McHutchinson JG, Gordon SC et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958–965. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

