Analytical Performance Characteristics of the Cepheid GeneXpert Ebola Assay for the Detection of Ebola Virus
- PMID: 26562786
- PMCID: PMC4643052
- DOI: 10.1371/journal.pone.0142216
Analytical Performance Characteristics of the Cepheid GeneXpert Ebola Assay for the Detection of Ebola Virus
Erratum in
-
Correction: Analytical Performance Characteristics of the Cepheid GeneXpert Ebola Assay for the Detection of Ebola Virus.PLoS One. 2015 Dec 21;10(12):e0145896. doi: 10.1371/journal.pone.0145896. eCollection 2015. PLoS One. 2015. PMID: 26690909 Free PMC article. No abstract available.
Abstract
Background: The recently developed Xpert® Ebola Assay is a novel nucleic acid amplification test for simplified detection of Ebola virus (EBOV) in whole blood and buccal swab samples. The assay targets sequences in two EBOV genes, lowering the risk for new variants to escape detection in the test. The objective of this report is to present analytical characteristics of the Xpert® Ebola Assay on whole blood samples.
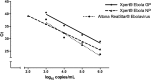
Methods and findings: This study evaluated the assay's analytical sensitivity, analytical specificity, inclusivity and exclusivity performance in whole blood specimens. EBOV RNA, inactivated EBOV, and infectious EBOV were used as targets. The dynamic range of the assay, the inactivation of virus, and specimen stability were also evaluated. The lower limit of detection (LoD) for the assay using inactivated virus was estimated to be 73 copies/mL (95% CI: 51-97 copies/mL). The LoD for infectious virus was estimated to be 1 plaque-forming unit/mL, and for RNA to be 232 copies/mL (95% CI 163-302 copies/mL). The assay correctly identified five different Ebola viruses, Yambuku-Mayinga, Makona-C07, Yambuku-Ecran, Gabon-Ilembe, and Kikwit-956210, and correctly excluded all non-EBOV isolates tested. The conditions used by Xpert® Ebola for inactivation of infectious virus reduced EBOV titer by ≥6 logs.
Conclusion: In summary, we found the Xpert® Ebola Assay to have high analytical sensitivity and specificity for the detection of EBOV in whole blood. It offers ease of use, fast turnaround time, and remote monitoring. The test has an efficient viral inactivation protocol, fulfills inclusivity and exclusivity criteria, and has specimen stability characteristics consistent with the need for decentralized testing. The simplicity of the assay should enable testing in a wide variety of laboratory settings, including remote laboratories that are not capable of performing highly complex nucleic acid amplification tests, and during outbreaks where time to detection is critical.
Conflict of interest statement
Figures
References
-
- Kuhn JH, Andersen KG, Baize S, Bao Y, Bavari S, Berthet N, et al. Nomenclature- and database-compatible names for the two Ebola virus variants that emerged in Guinea and the Democratic Republic of the Congo in 2014. Viruses. 2014;6(11):4760–99. Epub 2014/11/26. 10.3390/v6114760 ; PubMed Central PMCID: PMCPmc4246247. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

