Cryptosporidium Lactate Dehydrogenase Is Associated with the Parasitophorous Vacuole Membrane and Is a Potential Target for Developing Therapeutics
- PMID: 26562790
- PMCID: PMC4642935
- DOI: 10.1371/journal.ppat.1005250
Cryptosporidium Lactate Dehydrogenase Is Associated with the Parasitophorous Vacuole Membrane and Is a Potential Target for Developing Therapeutics
Abstract
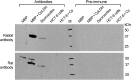
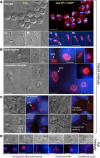
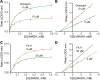
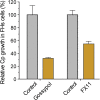
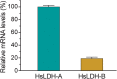
The apicomplexan, Cryptosporidium parvum, possesses a bacterial-type lactate dehydrogenase (CpLDH). This is considered to be an essential enzyme, as this parasite lacks the Krebs cycle and cytochrome-based respiration, and mainly-if not solely, relies on glycolysis to produce ATP. Here, we provide evidence that in extracellular parasites (e.g., sporozoites and merozoites), CpLDH is localized in the cytosol. However, it becomes associated with the parasitophorous vacuole membrane (PVM) during the intracellular developmental stages, suggesting involvement of the PVM in parasite energy metabolism. We characterized the biochemical features of CpLDH and observed that, at lower micromolar levels, the LDH inhibitors gossypol and FX11 could inhibit both CpLDH activity (Ki = 14.8 μM and 55.6 μM, respectively), as well as parasite growth in vitro (IC50 = 11.8 μM and 39.5 μM, respectively). These observations not only reveal a new function for the poorly understood PVM structure in hosting the intracellular development of C. parvum, but also suggest LDH as a potential target for developing therapeutics against this opportunistic pathogen, for which fully effective treatments are not yet available.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Chen XM, Keithly JS, Paya CV, LaRusso NF. Cryptosporidiosis. New England J Med. 2002;346(22):1723–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

