Long-Term Benefit of Mesalamine Granules for Patients Who Achieved Corticosteroid-Induced Ulcerative Colitis Remission
- PMID: 26563167
- PMCID: PMC4700064
- DOI: 10.1007/s10620-015-3866-7
Long-Term Benefit of Mesalamine Granules for Patients Who Achieved Corticosteroid-Induced Ulcerative Colitis Remission
Abstract
Background: Patients with ulcerative colitis (UC) who achieve remission with corticosteroids often relapse after tapering or discontinuation; alternative treatments limiting steroid exposure and UC relapse would be beneficial. It remains uncertain whether patients with corticosteroid-induced remission experience benefit with mesalamine granules (MG), a locally acting aminosalicylate extended-release capsule formulation for maintenance of UC remission in adults.
Aims: Efficacy and safety of MG 1.5 g once daily was evaluated in patients with UC in corticosteroid-induced remission.
Methods: Data from patients with previous corticosteroid use to achieve baseline UC remission were analyzed from two 6-month randomized, double-blind, placebo-controlled trials and a 24-month open-label extension (OLE). Six-month relapse-free rates were assessed using the revised Sutherland Disease Activity Index. UC-related adverse events (AEs) were recorded during the 30 months.
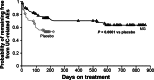
Results: Included were 158 steroid-treated patients in UC remission (MG, n = 105; placebo, n = 53) and 74/105 MG-treated patients who continued MG in the OLE. A significantly larger percentage of patients remained relapse-free at 6 months with MG (77.1 %) versus placebo (54.7 %; P = 0.006), with a 55 % reduction in relapse risk (hazard ratio [HR] 0.45; 95 % CI 0.25-0.79). There was a similar (49.2 %) reduction in risk of UC-related AEs at 6 months (HR 0.51; 95 % CI 0.31-0.84; P = 0.009) that was sustained during the OLE.
Conclusions: MG 1.5 g once daily administered for maintenance of corticosteroid-induced remission was associated with low risk of relapse and UC-related AEs. CLINICALTRIALS.GOV: NCT00744016, NCT00767728, and NCT00326209.
Keywords: Inflammatory bowel diseases; Mesalamine; Remission; Steroids; Ulcerative colitis.
Figures


References
-
- Langan RC, Gotsch PB, Krafczyk MA, Skillinge DD. Ulcerative colitis: diagnosis and treatment. Am Fam Physician. 2007;76:1323–1330. - PubMed
-
- Kornbluth A, Sachar DB, The Practice Parameters Committee of the American College of Gastroenterology Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501–523. doi: 10.1038/ajg.2009.727. - DOI - PubMed
-
- Everhart JE, Ed. The Burden of Digestive Diseases in the United States. Washington, DC: United States Department of Health and Human Services; Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2008. http://www2.niddk.nih.gov/NR/rdonlyres/0B814CEA-222A-4677-9B38-89D3BC34E.... Accessed February 12, 2013. Report no. NIH Publication No. 09-6443.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

