Isolation and Potential for Transmission of Mycobacterium bovis at Human-livestock-wildlife Interface of the Serengeti Ecosystem, Northern Tanzania
- PMID: 26563417
- PMCID: PMC5434928
- DOI: 10.1111/tbed.12445
Isolation and Potential for Transmission of Mycobacterium bovis at Human-livestock-wildlife Interface of the Serengeti Ecosystem, Northern Tanzania
Abstract
Mycobacterium bovis, the causative agent of bovine tuberculosis (bTB), is a multihost pathogen of public health and veterinary importance. We characterized the M. bovis isolated at the human-livestock-wildlife interface of the Serengeti ecosystem to determine the epidemiology and risk of cross-species transmission between interacting hosts species. DNA was extracted from mycobacterial cultures obtained from sputum samples of 472 tuberculosis (TB) suspected patients and tissue samples from 606 livestock and wild animal species. M. bovis isolates were characterized using spoligotyping and Mycobacterial Interspersed Repetitive Units-Variable Tandem Repeats (MIRU-VNTR) on 24 loci. Only 5 M. bovis were isolated from the cultured samples. Spoligotyping results revealed that three M. bovis isolates from two buffaloes (Syncerus caffer) and 1 African civet (Civettictis civetta) belonged to SB0133 spoligotype. The two novel strains (AR1 and AR2) assigned as spoligotype SB2290 and SB2289, respectively, were identified from indigenous cattle (Bos indicus). No M. bovis was detected from patients with clinical signs consistent with TB. Of the 606 animal tissue specimens and sputa of 472 TB-suspected patients 43 (7.09%) and 12 (2.9%), respectively, yielded non-tuberculous mycobacteria (NTM), of which 20 isolates were M. intracellulare. No M. avium was identified. M. bovis isolates from wildlife had 45.2% and 96.8% spoligotype pattern agreement with AR1 and AR2 strains, respectively. This finding indicates that bTB infections in wild animals and cattle were epidemiologically related. Of the 24 MIRU-VNTR loci, QUB 11b showed the highest discrimination among the M. bovis strains. The novel strains obtained in this study have not been previously reported in the area, but no clear evidence for recent cross-species transmission of M. bovis was found between human, livestock and wild animals.
Keywords: Mycobacterium bovis; MIRU-VNTR; human-animal interface; serengeti ecosystem; spoligotype.
© 2015 The Authors. Transboundary and Emerging Diseases Published by Blackwell Verlag GmbH.
Figures


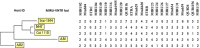
References
-
- Berg, S. , Garcia‐Pelayo M. C., Muller B., Hailu E., Asiimwe B., Kremer K., Dale J., Boniotti M. B., Rodriguez S., Hilty M., Rigouts L., Firdessa R., Machado A., Mucavele C., Ngandolo B. N. R., Bruchfeld J., Boschiroli L., Müller A., Sahraoui N., Pacciarini M., Cadmus S., Joloba M., van Soolingen D., Michel A. L., Djønne B., Aranaz A., Zinsstag J., van Helden P. D., Portaels F., Kazwala R., Källenius G., Aseffa R. G. H. A., and Gordon S., 2011: African 2, a clonal complex of Mycobacterium bovis epidemiologically important in East Africa. J. Bacteriol. 193, 670. - PMC - PubMed
-
- Christianson, S. , Wolfe J., Orr P., Karlowsky J., Levett P. N., Horsman G. B., Thibert L., Tang P., and Sharma M. K., 2009: Evaluation of 24 locus MIRU‐VNTR genotyping of Mycobacterium tuberculosis isolates in Canada. Tuberculosis 90, 31–38. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

