Genetics on the Fly: A Primer on the Drosophila Model System
- PMID: 26564900
- PMCID: PMC4649653
- DOI: 10.1534/genetics.115.183392
Genetics on the Fly: A Primer on the Drosophila Model System
Abstract
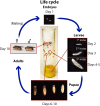
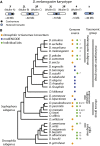
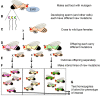
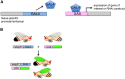
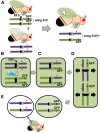
Fruit flies of the genus Drosophila have been an attractive and effective genetic model organism since Thomas Hunt Morgan and colleagues made seminal discoveries with them a century ago. Work with Drosophila has enabled dramatic advances in cell and developmental biology, neurobiology and behavior, molecular biology, evolutionary and population genetics, and other fields. With more tissue types and observable behaviors than in other short-generation model organisms, and with vast genome data available for many species within the genus, the fly's tractable complexity will continue to enable exciting opportunities to explore mechanisms of complex developmental programs, behaviors, and broader evolutionary questions. This primer describes the organism's natural history, the features of sequenced genomes within the genus, the wide range of available genetic tools and online resources, the types of biological questions Drosophila can help address, and historical milestones.
Keywords: Drosophila; comparative genomics; development; model organism.
Copyright © 2015 by the Genetics Society of America.
Figures


References
-
- Adams M. D., Celniker S. E., Holt R. A., Evans C. A., Gocayne J. D., et al. , 2000. The genome sequence of Drosophila melanogaster. Science 287: 2185–2195. - PubMed
-
- Adryan B., Teichmann S. A., 2006. FlyTF: a systematic review of site-specific transcription factors in the fruit fly Drosophila melanogaster. Bioinformatics 22: 1532–1533. - PubMed
-
- Armknecht S., Boutros M., Kiger A., Nybakken K., Mathey-Prevot B., et al. , 2005. High-throughput RNA interference screens in Drosophila tissue culture cells. Methods Enzymol. 392: 55–73. - PubMed
-
- Arnone M. I., Davidson E. H., 1997. The hardwiring of development: organization and function of genomic regulatory systems. Development 124: 1851–1864. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

