Intact Heart Loose Patch Photolysis Reveals Ionic Current Kinetics During Ventricular Action Potentials
- PMID: 26565013
- PMCID: PMC4851170
- DOI: 10.1161/CIRCRESAHA.115.307399
Intact Heart Loose Patch Photolysis Reveals Ionic Current Kinetics During Ventricular Action Potentials
Abstract
Rationale: Assessing the underlying ionic currents during a triggered action potential (AP) in intact perfused hearts offers the opportunity to link molecular mechanisms with pathophysiological problems in cardiovascular research. The developed loose patch photolysis technique can provide striking new insights into cardiac function at the whole heart level during health and disease.
Objective: To measure transmembrane ionic currents during an AP to determine how and when surface Ca(2+) influx that triggers Ca(2+)-induced Ca(2+) release occurs and how Ca(2+)-activated conductances can contribute to the genesis of AP phase 2.
Methods and results: Loose patch photolysis allows the measurement of transmembrane ionic currents in intact hearts. During a triggered AP, a voltage-dependent Ca(2+) conductance was fractionally activated (dis-inhibited) by rapidly photo-degrading nifedipine, the Ca(2+) channel blocker. The ionic currents during a mouse ventricular AP showed a fast early component and a slower late component. Pharmacological studies established that the molecular basis underlying the early component was driven by an influx of Ca(2+) through the L-type channel, CaV 1.2. The late component was identified as an Na(+)-Ca(2+) exchanger current mediated by Ca(2+) released from the sarcoplasmic reticulum.
Conclusions: The novel loose patch photolysis technique allowed the dissection of transmembrane ionic currents in the intact heart. We were able to determine that during an AP, L-type Ca(2+) current contributes to phase 1, whereas Na(+)-Ca(2+) exchanger contributes to phase 2. In addition, loose patch photolysis revealed that the influx of Ca(2+) through L-type Ca(2+) channels terminates because of voltage-dependent deactivation and not by Ca(2+)-dependent inactivation, as commonly believed.
Keywords: action potentials; calcium signaling; excitation contraction coupling; ionic currents; nifedipine; sarcoplasmic reticulum.
© 2015 American Heart Association, Inc.
Figures

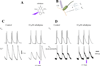
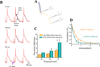







Comment in
-
From Single Myocyte to Whole Heart: The Intricate Dance of Electrophysiology and Modeling.Circ Res. 2016 Jan 22;118(2):184-6. doi: 10.1161/CIRCRESAHA.115.308067. Circ Res. 2016. PMID: 26838308 Free PMC article. No abstract available.
References
-
- Higuchi T, Nakaya Y. T wave polarity related to the repolarization process of epicardial and endocardial ventricular surfaces. Am Heart J. 1984;108:290–295. - PubMed
-
- Woodbury LA, Hecht HH, Christopherson AR. Membrane resting and action potentials of single cardiac muscle fibers of the frog ventricle. Am J Physiol. 1951;164:307–318. - PubMed
-
- Macfarlane WV. The plateau of the action potential of the frog ventricle. Circ Res. 1960;8:47–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

