Durable Clinical Response to Entrectinib in NTRK1-Rearranged Non-Small Cell Lung Cancer
- PMID: 26565381
- PMCID: PMC4643748
- DOI: 10.1097/01.JTO.0000473485.38553.f0
Durable Clinical Response to Entrectinib in NTRK1-Rearranged Non-Small Cell Lung Cancer
Abstract
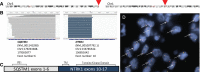
Introduction: Chromosomal rearrangements involving neurotrophic tyrosine kinase 1 (NTRK1) occur in a subset of non-small cell lung cancers (NSCLCs) and other solid tumor malignancies, leading to expression of an oncogenic TrkA fusion protein. Entrectinib (RXDX-101) is an orally available tyrosine kinase inhibitor, including TrkA. We sought to determine the frequency of NTRK1 rearrangements in NSCLC and to assess the clinical activity of entrectinib.
Methods: We screened 1378 cases of NSCLC using anchored multiplex polymerase chain reaction (AMP). A patient with an NTRK1 gene rearrangement was enrolled onto a Phase 1 dose escalation study of entrectinib in adult patients with locally advanced or metastatic tumors (NCT02097810). We assessed safety and response to treatment.
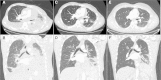
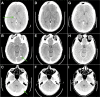
Results: We identified NTRK1 gene rearrangements at a frequency of 0.1% in this cohort. A patient with stage IV lung adenocrcinoma with an SQSTM1-NTRK1 fusion transcript expression was treated with entrectinib. Entrectinib was well tolerated, with no grade 3-4 adverse events. Within three weeks of starting on treatment, the patient reported resolution of prior dyspnea and pain. Restaging CT scans demonstrated a RECIST partial response (PR) and complete resolution of all brain metastases. This patient has continued on treatment for over 6 months with an ongoing PR.
Conclusions: Entrectinib demonstrated significant anti-tumor activity in a patient with NSCLC harboring an SQSTM1-NTRK1 gene rearrangement, indicating that entrectinib may be an effective therapy for tumors with NTRK gene rearrangements, including those with central nervous system metastases.
Conflict of interest statement
Disclosure: AFF has received consultant fees from Intervention Insights. LPL has received consultant fees from and has equity in ArcherDx. AD has received honoraria and travel expenses, and has served on the speaker’s Bureau for Ignyta, Inc. DJ has received consultant fees from Genentech and Celgene. DBC has received honoraria and consultant fees from Pfizer, honoraria from Boehringer Ingelheim, and consultant fees from Aria Pharmaceuticals. PSM, GGL, ZH, EC-M, DL and JL are employees of and have equity in Ignyta, Inc. AJI has received consultant fees and has equity in ArcherDx, and has received consulting fees from Chugai, Constellation, and DebioPharm. ATS has received consultant fees or served on the advisory board for Ignyta, Inc., Pfizer, Novartis, Genentech, Roche, Blueprint Medicine, and EMD Serono. ZZ, AM, MP, TMB, SVL, and S-HIO declare no conflicts of interest.
Figures

References
-
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4(4):299–309. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

