A Phase 1 Trial to Assess the Safety, Acceptability, Pharmacokinetics, and Pharmacodynamics of a Novel Dapivirine Vaginal Film
- PMID: 26565716
- PMCID: PMC5040830
- DOI: 10.1097/QAI.0000000000000897
A Phase 1 Trial to Assess the Safety, Acceptability, Pharmacokinetics, and Pharmacodynamics of a Novel Dapivirine Vaginal Film
Abstract
Background: Films may deliver antiretroviral drugs efficiently to mucosal tissues. In this first in-human trial of a vaginal film for delivering the nonnucleoside reverse transcriptase inhibitor dapivirine, safety, pharmacokinetics, and pharmacodynamics of film and gel formulations were compared with placebo.
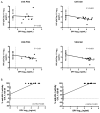
Methods: Sixty-one healthy HIV-negative women were randomized to daily dapivirine (0.05%) or placebo gel, or dapivirine (1.25 mg) or placebo film for seven days. The proportion of participants experiencing grade 2 and higher adverse events related to study product were compared. Plasma dapivirine concentrations were quantified. Paired cervical and vaginal tissue biopsies obtained ∼2 hours after the last dose were measured for tissue drug concentration and exposed to HIV in an ex vivo challenge assay.
Results: Two grade 2 related adverse events occurred in the placebo film group. Women randomized to gel and film products had 4 log10 higher of dapivirine in cervical and vaginal tissues than plasma. Although gel and film users had comparable plasma dapivirine concentrations, tissue concentrations of dapivirine were 3-5 times higher in the gel users when compared with film users. HIV replication in the ex vivo challenge assay was significantly reduced in vaginal tissues from women randomized to dapivirine film or gel; furthermore, tissue drug concentrations were highly correlated with HIV protection. Women rated the film more comfortable with less leakage but found it more difficult to insert than gel.
Discussion: Both film and gel delivered dapivirine at concentrations sufficient to block HIV ex vivo. This proof-of-concept study suggests film formulations for microbicides merit further investigation.
Figures

References
-
- Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012 Aug 2;367(5):423–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

