Specific Binding of Tetratricopeptide Repeat Proteins to Heat Shock Protein 70 (Hsp70) and Heat Shock Protein 90 (Hsp90) Is Regulated by Affinity and Phosphorylation
- PMID: 26565746
- PMCID: PMC4714923
- DOI: 10.1021/acs.biochem.5b00801
Specific Binding of Tetratricopeptide Repeat Proteins to Heat Shock Protein 70 (Hsp70) and Heat Shock Protein 90 (Hsp90) Is Regulated by Affinity and Phosphorylation
Abstract
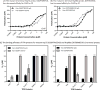
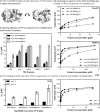
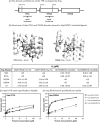
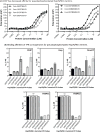
Heat shock protein 70 (Hsp70) and heat shock protein 90 (Hsp90) require the help of tetratricopeptide repeat (TPR) domain-containing cochaperones for many of their functions. Each monomer of Hsp70 or Hsp90 can interact with only a single TPR cochaperone at a time, and each member of the TPR cochaperone family brings distinct functions to the complex. Thus, competition for TPR binding sites on Hsp70 and Hsp90 appears to shape chaperone activity. Recent structural and biophysical efforts have improved our understanding of chaperone-TPR contacts, focusing on the C-terminal EEVD motif that is present in both chaperones. To better understand these important protein-protein interactions on a wider scale, we measured the affinity of five TPR cochaperones, CHIP, Hop, DnaJC7, FKBP51, and FKBP52, for the C-termini of four members of the chaperone family, Hsc70, Hsp72, Hsp90α, and Hsp90β, in vitro. These studies identified some surprising selectivity among the chaperone-TPR pairs, including the selective binding of FKBP51/52 to Hsp90α/β. These results also revealed that other TPR cochaperones are only able to weakly discriminate between the chaperones or between their paralogs. We also explored whether mimicking phosphorylation of serine and threonine residues near the EEVD motif might impact affinity and found that pseudophosphorylation had selective effects on binding to CHIP but not other cochaperones. Together, these findings suggest that both intrinsic affinity and post-translational modifications tune the interactions between the Hsp70 and Hsp90 proteins and the TPR cochaperones.
Figures


References
-
- Chiang HL, Terlecky SR, Plant CP, Dice JF. A Role for a 70-Kilodaton Heat-Shock Protein in Lysosomal Degradation of Intracellular Proteins. Science. 1989;246:382–385. - PubMed
-
- Bercovich B, Stancovski I, Mayer A, Blumenfeld N, Laszlo A, Schwartz AL, Ciechanover A. Ubiquitin-dependent degradation of certain protein substrates in vitro requires the molecular chaperone Hsc70. J Biol Chem. 1997;272:9002–9010. - PubMed
-
- Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451. - PubMed
-
- Hartl FU, Hayer-Hartl M. Protein folding – Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

