Exosome Secretion by the Parasitic Protozoan Leishmania within the Sand Fly Midgut
- PMID: 26565909
- PMCID: PMC4644496
- DOI: 10.1016/j.celrep.2015.09.058
Exosome Secretion by the Parasitic Protozoan Leishmania within the Sand Fly Midgut
Abstract
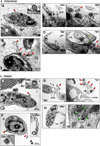
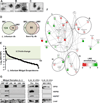
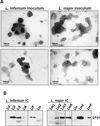
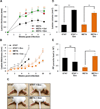
Despite several studies describing the secretion of exosomes by Leishmania in vitro, observation of their formation and release in vivo has remained a major challenge. Herein, we show that Leishmania constitutively secretes exosomes within the lumen of the sand fly midgut through a mechanism homologous to the mammalian pathway. Through egestion experiments, we demonstrate that Leishmania exosomes are part of the sand fly inoculum and are co-egested with the parasite during the insect's bite, possibly influencing the host infectious process. Indeed, co-inoculation of mice footpads with L. major plus midgut-isolated or in-vitro-isolated L. major exosomes resulted in a significant increase in footpad swelling. Notably, co-injections produced exacerbated lesions through overinduction of inflammatory cytokines, in particular IL-17a. Our data indicate that Leishmania exosomes are an integral part of the parasite's infectious life cycle, and we propose to add these vesicles to the repertoire of virulence factors associated with vector-transmitted infections.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Bendtsen JD, Jensen LJ, Blom N, Von Heijne G, Brunak S. Feature-based prediction of non-classical and leaderless protein secretion. Protein engineering, design & selection : PEDS. 2004;17:349–356. - PubMed
-
- Boaventura VS, Santos CS, Cardoso CR, de Andrade J, Dos Santos WL, Clarencio J, Silva JS, Borges VM, Barral-Netto M, Brodskyn CI, et al. Human mucosal leishmaniasis: neutrophils infiltrate areas of tissue damage that express high levels of Th17-related cytokines. European journal of immunology. 2010;40:2830–2836. - PubMed
-
- Bobrie A, Colombo M, Raposo G, Thery C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–1668. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

