Bacterial chromosome organization and segregation
- PMID: 26566111
- PMCID: PMC4706359
- DOI: 10.1146/annurev-cellbio-100814-125211
Bacterial chromosome organization and segregation
Abstract
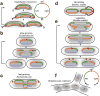
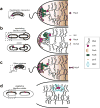
If fully stretched out, a typical bacterial chromosome would be nearly 1 mm long, approximately 1,000 times the length of a cell. Not only must cells massively compact their genetic material, but they must also organize their DNA in a manner that is compatible with a range of cellular processes, including DNA replication, DNA repair, homologous recombination, and horizontal gene transfer. Recent work, driven in part by technological advances, has begun to reveal the general principles of chromosome organization in bacteria. Here, drawing on studies of many different organisms, we review the emerging picture of how bacterial chromosomes are structured at multiple length scales, highlighting the functions of various DNA-binding proteins and the impact of physical forces. Additionally, we discuss the spatial dynamics of chromosomes, particularly during their segregation to daughter cells. Although there has been tremendous progress, we also highlight gaps that remain in understanding chromosome organization and segregation.
Keywords: Hi-C; ParA-ParB-parS; macrodomains; nucleoid-associated proteins; supercoiling; transcription.
Figures



References
-
- Adams D, Shekhtman E, Zechiedrich E, Schmid M, Cozzarelli N. The role of topoisomerase-IV in partitioning bacterial replicons and the structure of catenated intermediates in DNA replication. Cell. 1992;71(2):277–88. - PubMed
-
- Auner H, Buckle M, Deufel A, Kutateladze T, Lazarus L, et al. Mechanism of transcriptional activation by FIS: role of core promoter structure and DNA topology. J Mol Biol. 2003;331(2):331–44. - PubMed
-
- Austin SJ, Mural RJ, Chattoraj DK, Abeles AL. Trans- and cis-acting elements for the replication of P1 miniplasmids. J Mol Biol. 1985;183(2):195–202. - PubMed
-
- Azam TA, Hiraga S, Ishihama A. Two types of localization of the DNA-binding proteins within the Escherichia coli nucleoid. Genes Cells. 2000;5(8):613–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

