The Association between Clinical Response to Ustekinumab and Immunogenicity to Ustekinumab and Prior Adalimumab
- PMID: 26566272
- PMCID: PMC4643875
- DOI: 10.1371/journal.pone.0142930
The Association between Clinical Response to Ustekinumab and Immunogenicity to Ustekinumab and Prior Adalimumab
Abstract
Background: Immunogenicity due to antidrug antibodies (ADA) to tumor necrosis factor (TNF)-α antagonists is known to decrease treatment response. However, few studies have investigated ADA in ustekinumab, an interleukin-12 and -23 antagonist, in a clinical setting. This study aimed to investigate the immunogenicity of ustekinumab and its clinical consequences in psoriasis.
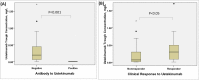
Methods: This prospective observational study enrolled 76 patients with plaque psoriasis who were treated with ustekinumab for a minimum of 7 months. Blood samples were drawn just prior to scheduled ustekinumab injection during clinic visits. Levels of anti-ustekinumab antibody (AUA) and serum ustekinumab concentration were measured respectively by radioimmunoassays and enzyme-linked immunoassays respectively, and correlated to clinical data and Psoriasis Area and Severity Index (PASI).
Results: AUA was detected in 6.5% of patients after a mean of 13 months of treatment. Patients with positive AUA had significantly lower serum ustekinumab concentrations (0.01 vs. 0.2 mg/L, p<0.001) and lower PASI 50 response than patients without AUA (0% vs. 69%, p = 0.004).The percentage of AUA formation was comparable between patients who had failed previous adalimumab with or without anti-adalimumab antibodies (AAA) (14.3% vs. 12.5%, p = 1.00). However, a higher proportion of switchers without AAA obtaining PASI50 (71.4% vs. 37.5%) and PASI75 response (42.9% vs.12.5%) within 7 months of ustekinumab treatment than with AAA though this difference did not reach statistical significance.
Conclusions: Our results suggest that presence of AUA was significantly associated with treatment failure for ustekinumab, though limited by a small sample size. Also, determining the presence of ADA to antecedent TNF-α antagonists may assist in choosing an optimized subsequent treatment modality achieving treatment success.
Conflict of interest statement
Figures
References
-
- Chiu H-Y, Cheng Y-P, Tsai T-F. (2012) T helper type 17 in psoriasis: From basic immunology to clinical practice. Dermatologica Sinica 30: 136–141.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

