The role of brimonidine tartrate gel in the treatment of rosacea
- PMID: 26566370
- PMCID: PMC4627400
- DOI: 10.2147/CCID.S58920
The role of brimonidine tartrate gel in the treatment of rosacea
Abstract
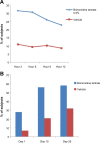
Rosacea is a chronic cutaneous condition with a prevalence rate ranging from 9.6% to 22% in recent studies. Facial erythema (transient and permanent) is considered a common denominator that is frequently observed in all subtypes of rosacea and is estimated to affect more than 40 million people worldwide. Brimonidine tartrate is a selective α2-adrenergic receptor agonist and is the first topical treatment approved for facial erythema of rosacea. Clinical trials have demonstrated that brimonidine tartrate provided significantly greater efficacy, compared to vehicle, for the treatment of moderate to severe erythema of rosacea. In addition, brimonidine tartrate has demonstrated a rapid onset of effect, duration of action throughout the day, and good safety profile in studies of up to 1 year. This review critically discusses the role of brimonidine tartrate for the treatment of facial erythema of rosacea by examining both clinical study data and real-world dermatologist experiences across a wide spectrum of treated patients, and concludes that it is a significant therapeutic option in the management of an unmet need of this chronic condition.
Keywords: adverse event; brimonidine tartrate; erythema; rosacea.
Figures


References
-
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: Report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46(4):584–587. - PubMed
-
- Elewski BE, Draelos Z, Dréno B, Jansen T, Layton A, Picardo M. Rosacea – global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25(2):188–200. - PubMed
-
- Tan J, Blume-Peytavi U, Ortonne JP, et al. An observational cross-sectional survey of rosacea: clinical associations and progression between subtypes. Br J Dermatol. 2013;169(3):555–562. - PubMed
-
- Drake L, editor. Rosacea Review. Barrington, IL: National Rosacea Society; 2000. [Accessed April 16, 2014]. Patients over 50 hardest hit with rosacea symptoms. webpage on the Internet. Available from: http://www.rosacea.org/rr/2000/fall/article_2.php.
-
- Blount BW, Pelletier AL. Rosacea: a common, yet commonly overlooked, condition. Am Fam Physician. 2002;66(3):435–440. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

