Effect of genetic background on the dystrophic phenotype in mdx mice
- PMID: 26566673
- PMCID: PMC4690497
- DOI: 10.1093/hmg/ddv460
Effect of genetic background on the dystrophic phenotype in mdx mice
Abstract
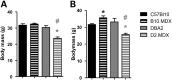
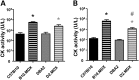
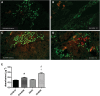
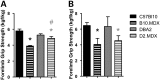
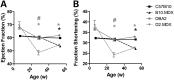
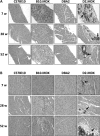
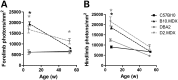
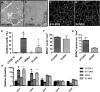
Genetic background significantly affects phenotype in multiple mouse models of human diseases, including muscular dystrophy. This phenotypic variability is partly attributed to genetic modifiers that regulate the disease process. Studies have demonstrated that introduction of the γ-sarcoglycan-null allele onto the DBA/2J background confers a more severe muscular dystrophy phenotype than the original strain, demonstrating the presence of genetic modifier loci in the DBA/2J background. To characterize the phenotype of dystrophin deficiency on the DBA/2J background, we created and phenotyped DBA/2J-congenic Dmdmdx mice (D2-mdx) and compared them with the original, C57BL/10ScSn-Dmdmdx (B10-mdx) model. These strains were compared with their respective control strains at multiple time points between 6 and 52 weeks of age. Skeletal and cardiac muscle function, inflammation, regeneration, histology and biochemistry were characterized. We found that D2-mdx mice showed significantly reduced skeletal muscle function as early as 7 weeks and reduced cardiac function by 28 weeks, suggesting that the disease phenotype is more severe than in B10-mdx mice. In addition, D2-mdx mice showed fewer central myonuclei and increased calcifications in the skeletal muscle, heart and diaphragm at 7 weeks, suggesting that their pathology is different from the B10-mdx mice. The new D2-mdx model with an earlier onset and more pronounced dystrophy phenotype may be useful for evaluating therapies that target cardiac and skeletal muscle function in dystrophin-deficient mice. Our data align the D2-mdx with Duchenne muscular dystrophy patients with the LTBP4 genetic modifier, making it one of the few instances of cross-species genetic modifiers of monogenic traits.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures



References
-
- Hatzipetros T., Bogdanik L.P., Tassinari V.R., Kidd J.D., Moreno A.J., Davis C., Osborne M., Austin A., Vieira F.G., Lutz C. (2014) C57BL/6J congenic Prp-TDP43A315T mice develop progressive neurodegeneration in the myenteric plexus of the colon without exhibiting key features of ALS. Brain Res., 1584, 59–72. - PubMed
-
- Heiman-Patterson T.D., Sher R.B., Blankenhorn E.A., Alexander G., Deitch J.S., Kunst C.B., Maragakis N., Cox G. (2011) Effect of genetic background on phenotype variability in transgenic mouse models of amyotrophic lateral sclerosis: a window of opportunity in the search for genetic modifiers. Amyotroph. Lateral Scler., 12, 79–86. - PubMed
-
- Sicinski P., Geng Y., Ryder-Cook A.S., Barnard E.A., Darlison M.G., Barnard P.J. (1989) The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science, 244, 1578–1580. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

