The histone demethylase KDM5A is a key factor for the resistance to temozolomide in glioblastoma
- PMID: 26566863
- PMCID: PMC4825557
- DOI: 10.1080/15384101.2015.1090063
The histone demethylase KDM5A is a key factor for the resistance to temozolomide in glioblastoma
Abstract
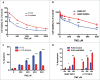
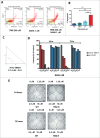
Notwithstanding current multimodal treatment, including surgery, radiotherapy and chemotherapy with temozolomide (TMZ), median survival of glioblastoma (GBM) patients is about 14 months, due to the rapid emergence of cell clones resistant to treatment. Therefore, understanding the mechanisms underlying chemoresistance is mandatory to improve treatments' outcome. We generated TMZ resistant cells (TMZ-R) from a GBM cell line and from cancer stem cell-enriched cultures isolated from human GBMs. We demonstrated that TMZ resistance is partially reverted by "drug wash-out" suggesting the contribution of epigenetic mechanisms in drug resistance and supporting the possibility of TMZ rechallenge in GBM patients after prior drug exposure. The expression of histone lysine demethylase genes (KDMs) was increased in TMZ-R cells compared to parental cells, and TMZ resistance or restored sensitivity was mimicked by over-expressing or inactivating KDM5A. Methylation and expression of O6-methylguanine-DNA methyltransferase (MGMT) and drug efflux mechanisms were not altered in TMZ-R cells compared to parental TMZ sensitive cells. TMZ-R cells transiently acquired morphologic and molecular characteristics of differentiated tumor cells, features that were lost after drug wash-out. In conclusion, we demonstrated that treatment-induced TMZ resistance in GBM involves epigenetic mechanisms in a subset of slow-cycling and transiently partially differentiated cells that escape drug cytotoxicity, overcome G2 checkpoint and sustain clonal growth. We found that TMZ-R cells are sensitive to histone deacethylase inhibitors (HDACi) that synergize with TMZ. This strong synergism could be exploited to develop novel combined adjuvant therapies for this rapidly progressing and invariably lethal cancer.
Keywords: DNA methylation; KDM5A; cancer stem cells; epigenetic; glioblastoma; histone demethylase; temozolomide.
Figures





References
-
- Dunn GP, Rinne ML, Wykosky J, Genovese G, Quayle SN, Dunn IF, Agarwalla PK, Chheda MG, Campos B, Wang A, et al.. Emerging insights into the molecular and cellular basis of glioblastoma. Genes Dev 2012; 26:756-84; PMID:22508724; http://dx.doi.org/10.1101/gad.187922.112 - DOI - PMC - PubMed
-
- Dirks PB. Brain tumor stem cells: the cancer stem cell hypothesis writ large. Mol Oncol 2010; 4:420-30; PMID:20801091; http://dx.doi.org/10.1016/j.molonc.2010.08.001 - DOI - PMC - PubMed
-
- Yamada R, Nakano I. Glioma stem cells: their role in chemoresistance. World Neurosurg 2012; 77:237-40; PMID:22501017; http://dx.doi.org/10.1016/j.wneu.2012.01.004 - DOI - PubMed
-
- Florio T, Barbieri F. The status of the art of human malignant glioma management: the promising role of targeting tumor-initiating cells. Drug Discov Today 2012; 17:1103-10; PMID:22704957; http://dx.doi.org/10.1016/j.drudis.2012.06.001 - DOI - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al.. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352:987-96; PMID:15758009; http://dx.doi.org/10.1056/NEJMoa043330 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
