Quality of outcome reporting in phase II studies in pulmonary tuberculosis
- PMID: 26566930
- PMCID: PMC4644328
- DOI: 10.1186/s13063-015-1050-1
Quality of outcome reporting in phase II studies in pulmonary tuberculosis
Abstract
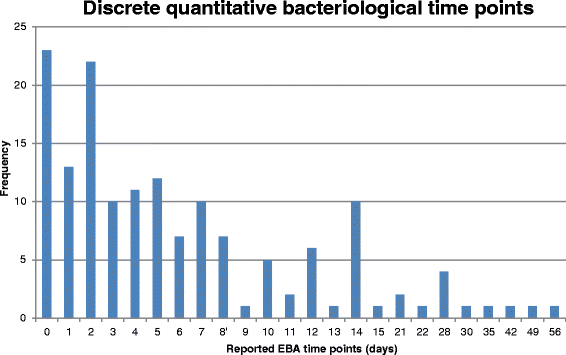
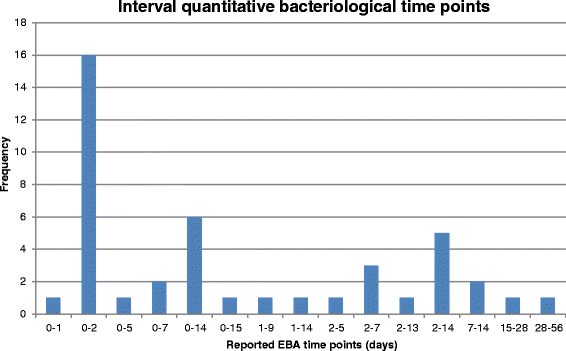
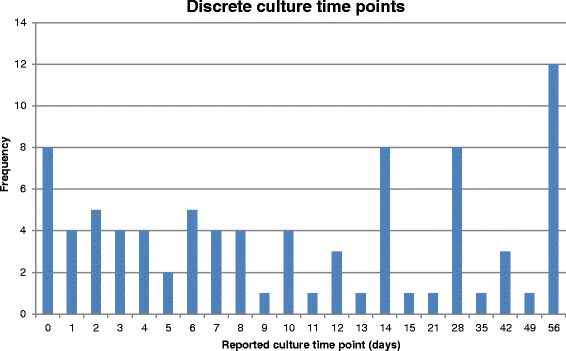
Tuberculosis (TB) remains a major killer amongst the infectious diseases. Current treatment involves a four-drug regimen for at least 6 months. New drugs and regimens are required to shorten treatment duration, reduce toxicity and combat drug resistance, but the optimal methodology to define the critical path for novel regimens is not well defined. We undertook a systematic review to summarise outcomes reported in Phase II trials of patients with newly diagnosed pulmonary TB to assess the need for a core outcome set. A systematic search of databases (PubMed, MEDLINE, EMBASE and LILACs) was conducted on 1 May 2015 to retrieve relevant peer-reviewed articles. Reference lists of included studies were also searched. This systematic review considered all reported outcomes. Risk of bias was considered via sequence generation, allocation concealment, blinding, reasons for exclusions, and selective reporting. Of 55 included studies, 20 were Phase IIB studies based on culture conversion, 32 were Phase IIA studies based on quantitative bacteriology, and three considered alternative outcomes. Large variation in reported outcomes and trial characteristics was observed across the included studies. Bacteriological results were as often expressed in terms of positivity as negativity, with varying definitions of culture conversion. Variation in reporting was particularly marked for Phase IIA studies, where multiple time intervals were typically selected for analysis and sometimes resulted in differing interpretations of the efficacy of drugs or regimens. Within both Phase IIA and IIB studies, there was variation in the time points at which the study participants were sampled, as well as in the bacteriological media and methods used. For successful future meta-analysis of early-phase studies, the findings of this review suggest that development of a core outcome set would be desirable. This would enable trial results to be more easily compared and combined, potentially leading to more effective development of new treatment strategies for patients with TB. Pending development of, and agreement on, such a core outcome set, we suggest some interim recommendations for reporting of future phase II studies of pulmonary tuberculosis.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

