Tumor-associated Endo180 requires stromal-derived LOX to promote metastatic prostate cancer cell migration on human ECM surfaces
- PMID: 26567111
- PMCID: PMC4761374
- DOI: 10.1007/s10585-015-9765-7
Tumor-associated Endo180 requires stromal-derived LOX to promote metastatic prostate cancer cell migration on human ECM surfaces
Abstract
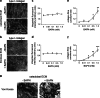
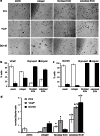
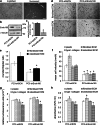
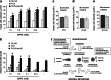
The diverse composition and structure of extracellular matrix (ECM) interfaces encountered by tumor cells at secondary tissue sites can influence metastatic progression. Extensive in vitro and in vivo data has confirmed that metastasizing tumor cells can adopt different migratory modes in response to their microenvironment. Here we present a model that uses human stromal cell-derived matrices to demonstrate that plasticity in tumor cell movement is controlled by the tumor-associated collagen receptor Endo180 (CD280, CLEC13E, KIAA0709, MRC2, TEM9, uPARAP) and the crosslinking of collagen fibers by stromal-derived lysyl oxidase (LOX). Human osteoblast-derived and fibroblast-derived ECM supported a rounded 'amoeboid-like' mode of cell migration and enhanced Endo180 expression in three prostate cancer cell lines (PC3, VCaP, DU145). Genetic silencing of Endo180 reverted PC3 cells from their rounded mode of migration towards a bipolar 'mesenchymal-like' mode of migration and blocked their translocation on human fibroblast-derived and osteoblast-derived matrices. The concomitant decrease in PC3 cell migration and increase in Endo180 expression induced by stromal LOX inhibition indicates that the Endo180-dependent rounded mode of prostate cancer cell migration requires ECM crosslinking. In conclusion, this study introduces a realistic in vitro model for the study of metastatic prostate cancer cell plasticity and pinpoints the cooperation between tumor-associated Endo180 and the stiff microenvironment imposed by stromal-derived LOX as a potential target for limiting metastatic progression in prostate cancer.
Keywords: Bone; Cell migration; Collagen; Fibroblast; Osteoblast; Prostate cancer.
Figures


References
-
- Sturge J, Caley MP, Waxman J. Bone metastasis in prostate cancer: emerging therapeutic strategies. Nat Rev Clin Oncol. 2011;8(6):357–368. - PubMed
-
- Reichert JC, Quent VMC, Burke LJ, Stansfield SH, Clements JA, Hutmacher DW. Mineralized human primary osteoblast matrices as a model system to analyse interactions of prostate cancer cells with the bone microenvironment. Biomaterials. 2010;31(31):7928–7936. doi: 10.1016/j.biomaterials.2010.06.055. - DOI - PubMed
-
- Hesami P, Holzapfel BM, Taubenberger A, Roudier M, Fazli L, Sieh S, Thibaudeau L, Gregory LS, Hutmacher DW, Clements JA. A humanized tissue-engineered in vivo model to dissect interactions between human prostate cancer cells and human bone. Clin Exp Metastasis. 2014 - PubMed
-
- Caley MP, Kogianni G, Adamarek A, Gronau JH, Rodriguez-Teja M, Fonseca AV, Mauri F, Sandison A, Rhim JS, Palmieri C, Cobb JP, Waxman J, Sturge J. TGFbeta1-Endo180-dependent collagen deposition is dysregulated at the tumour–stromal interface in bone metastasis. J Pathol. 2012;226(5):775–783. doi: 10.1002/path.3958. - DOI - PubMed
-
- Berning M, Pratzel-Wunder S, Bickenbach JR, Boukamp P. Three-dimensional in vitro skin and skin cancer models based on human fibroblast-derived matrix. Tissue Eng Part C Methods. 2015 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

