Impact of treatment with biologic DMARDs on the risk of sepsis or mortality after serious infection in patients with rheumatoid arthritis
- PMID: 26567181
- PMCID: PMC5013078
- DOI: 10.1136/annrheumdis-2015-207838
Impact of treatment with biologic DMARDs on the risk of sepsis or mortality after serious infection in patients with rheumatoid arthritis
Abstract
Objective: This observational cohort study investigated the impact of biological (b) disease-modifying antirheumatic drugs (DMARDs) on the outcomes of serious infections (SIs) in patients with rheumatoid arthritis.
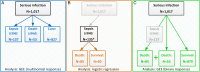
Methods: We investigated outcomes of SIs observed in 947 patients enrolled in the German biologics register RABBIT(Rheumatoid arthritis: observation of biologic therapy). Outcomes were (1) recovery without complication, (2) sepsis following SI (≤30 days), and (3) death after SI without known sepsis (≤90 days). We applied a multinomial generalised estimating equation model for longitudinal data to evaluate the risks of sepsis and death simultaneously.
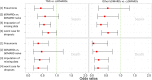
Results: Sepsis within 30 days after SI was reported in 135 out of 947 patients, 85 of these had a fatal outcome. Fifty-three patients died within 90 days after SI without known sepsis. The adjusted risk of developing sepsis increased with age and was higher in patients with chronic renal disease. Compared with conventional synthetic (cs)DMARDs, the risk was significantly lower when patients were exposed to bDMARDs at the time of SI (OR: 0.56, 95% CI 0.38 to 0.81). Risk factors of fatal SI were higher age, use of glucocorticoids at higher doses and heart failure. Patients treated with bDMARDs and those with better physical function had a significantly lower mortality risk.
Conclusions: These results suggest a beneficial effect of bDMARDs on the risk of sepsis after SI and the risk of a fatal outcome. Successful immunosuppression may prevent an unregulated host response to SI, that is, the escalation to sepsis. Further investigation is needed to validate these results.
Keywords: DMARDs (biologic); Epidemiology; Infections; Rheumatoid Arthritis; Treatment.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures
References
-
- Destarac LA, Ely EW. Sepsis in older patients: an emerging concern in critical care. Adv Sepsis 2001;2:15–22.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

