Structure and Properties of a Non-processive, Salt-requiring, and Acidophilic Pectin Methylesterase from Aspergillus niger Provide Insights into the Key Determinants of Processivity Control
- PMID: 26567911
- PMCID: PMC4714216
- DOI: 10.1074/jbc.M115.673152
Structure and Properties of a Non-processive, Salt-requiring, and Acidophilic Pectin Methylesterase from Aspergillus niger Provide Insights into the Key Determinants of Processivity Control
Abstract
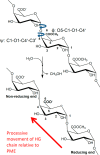
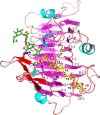
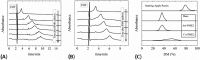
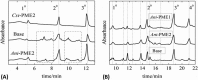
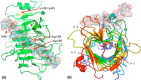
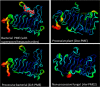
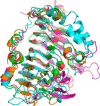
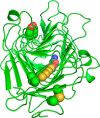
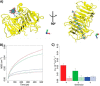
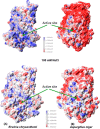
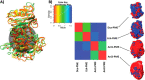
Many pectin methylesterases (PMEs) are expressed in plants to modify plant cell-wall pectins for various physiological roles. These pectins are also attacked by PMEs from phytopathogens and phytophagous insects. The de-methylesterification by PMEs of the O6-methyl ester groups of the homogalacturonan component of pectin, exposing galacturonic acids, can occur processively or non-processively, respectively, describing sequential versus single de-methylesterification events occurring before enzyme-substrate dissociation. The high resolution x-ray structures of a PME from Aspergillus niger in deglycosylated and Asn-linked N-acetylglucosamine-stub forms reveal a 10⅔-turn parallel β-helix (similar to but with less extensive loops than bacterial, plant, and insect PMEs). Capillary electrophoresis shows that this PME is non-processive, halophilic, and acidophilic. Molecular dynamics simulations and electrostatic potential calculations reveal very different behavior and properties compared with processive PMEs. Specifically, uncorrelated rotations are observed about the glycosidic bonds of a partially de-methyl-esterified decasaccharide model substrate, in sharp contrast to the correlated rotations of processive PMEs, and the substrate-binding groove is negatively not positively charged.
Keywords: capillary electrophoresis; carbohydrate processing; crystal structure; electrostatics; molecular dynamics; pectin methylesterase; processivity.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Atmodjo M. A., Hao Z., and Mohnen D. (2013) Evolving views of pectin biosynthesis. Annu. Rev. Plant Biol. 64, 747–779 - PubMed
-
- Caffall K. H., and Mohnen D. (2009) The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 344, 1879–1900 - PubMed
-
- Mohnen D. (2008) Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 11, 266–277 - PubMed
-
- Markovic O., and Janecek S. (2004) Pectin methylesterases: sequence-structural features and phylogenetic relationships. Carbohydr. Res. 339, 2281–2295 - PubMed
-
- Albersheim P., Darvill A., Roberts K., Sederoff R., and Staehelin A. (2010) Plant Cell Walls: From Chemistry to Biology, Garland Science, New York
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Research Materials

