IL-15 functions as a danger signal to regulate tissue-resident T cells and tissue destruction
- PMID: 26567920
- PMCID: PMC5079184
- DOI: 10.1038/nri3919
IL-15 functions as a danger signal to regulate tissue-resident T cells and tissue destruction
Abstract
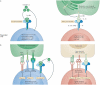
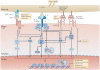
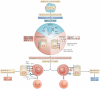
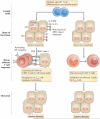
In this Opinion article, we discuss the function of tissues as a crucial checkpoint for the regulation of effector T cell responses, and the notion that interleukin-15 (IL-15) functions as a danger molecule that communicates to the immune system that the tissue is under attack and poises it to mediate tissue destruction. More specifically, we propose that expression of IL-15 in tissues promotes T helper 1 cell-mediated immunity and provides co-stimulatory signals to effector cytotoxic T cells to exert their effector functions and drive tissue destruction. Therefore, we think that IL-15 contributes to tissue protection by promoting the elimination of infected cells but that when its expression is chronically dysregulated, it can promote the development of complex T cell-mediated disorders associated with tissue destruction, such as coeliac disease and type 1 diabetes.
Figures

References
-
- Taylor GA, Feng CG, Sher A. Control of IFN-γ-mediated host resistance to intracellular pathogens by immunity-related GTPases (p47 GTPases) Microbes Infect. 2007;9:1644–1651. - PubMed
-
- Antonelli A, Ferrari SM, Corrado A, Di Domenicantonio A, Fallahi P. Autoimmune thyroid disorders. Autoimmun. Rev. 2015;14:174–180. - PubMed
-
- Jabri B, Sollid LM. Tissue-mediated control of immunopathology in coeliac disease. Nat. Rev. Immunol. 2009;9:858–870. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

