Twenty-eight-week results from the REALISTIC phase IIIb randomized trial: efficacy, safety and predictability of response to certolizumab pegol in a diverse rheumatoid arthritis population
- PMID: 26568428
- PMCID: PMC4644627
- DOI: 10.1186/s13075-015-0841-9
Twenty-eight-week results from the REALISTIC phase IIIb randomized trial: efficacy, safety and predictability of response to certolizumab pegol in a diverse rheumatoid arthritis population
Abstract
Introduction: This 28-week, phase IIIb study assessed safety and maintenance of response to certolizumab pegol (CZP) in a diverse population of rheumatoid arthritis (RA) patients, stratified by prior anti-TNF exposure, concomitant methotrexate (MTX) use and disease duration. The ability to predict achievement of low disease activity (LDA) at week 28 from improvements in Disease Activity Score 28 (DAS28), erythrocyte sedimentation rate (ESR), swollen joint count (SJC) and Clinical Disease Activity Index (CDAI) up to week 12 was assessed.
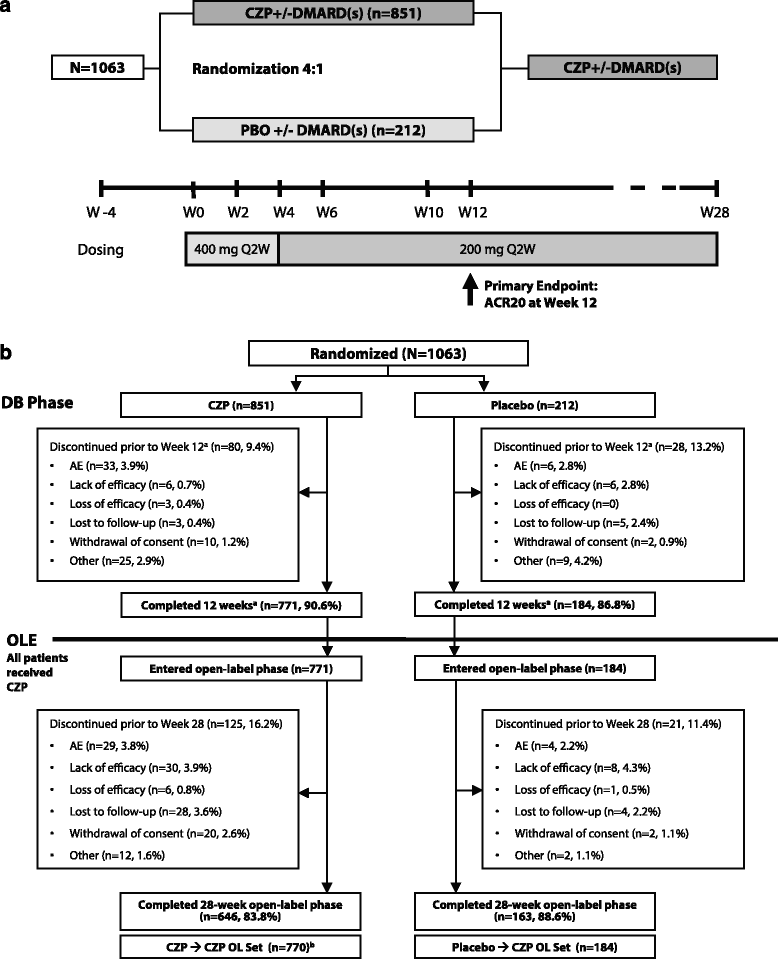
Methods: The 28-week study population included all patients who completed the double-blind (DB) phase and entered the open-label (OL) phase, receiving 200 mg CZP every 2 weeks (Q2W) ≥16 weeks. In the 12-week DB period, patients with active RA and an inadequate response to ≥1 disease-modifying antirheumatic drug (DMARD) were randomized 4:1 to CZP (400 mg at weeks 0, 2 and 4 then 200 mg Q2W) or placebo (Q2W), stratified by prior anti-TNF use, concomitant use of MTX and disease duration (<2 years vs. ≥2 years).
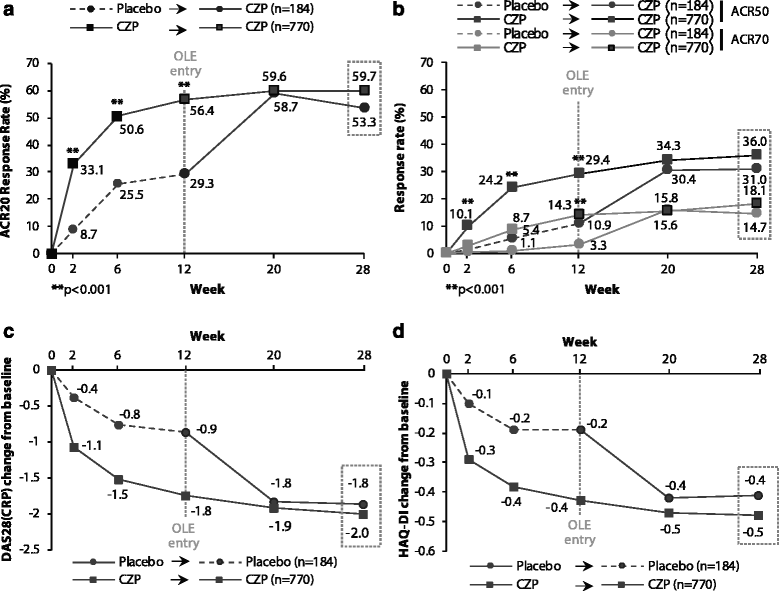
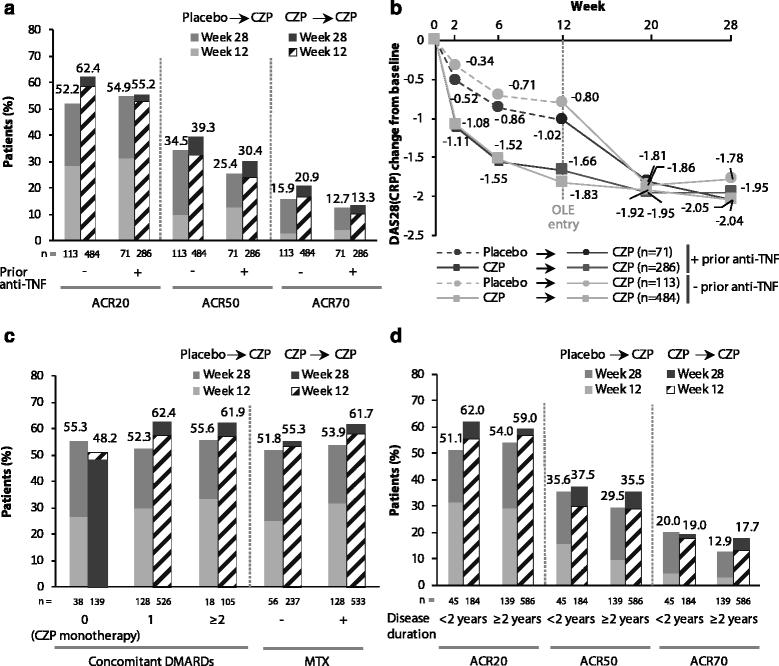
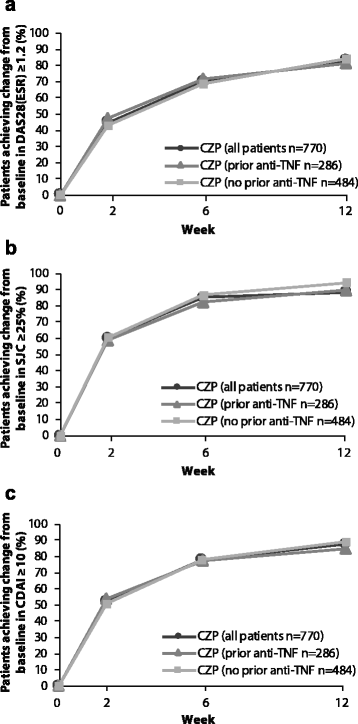
Results: A total of 955 patients entered the OL phase. At week 28, similar clinical improvements were seen in those receiving CZP throughout (CZP → CZP; n = 771) and those receiving placebo during the DB phase and switching to CZP in the OL phase (placebo → CZP; n = 184) (ACR20 response rate = 59.7% vs. 53.3%; ACR50/ACR70 response rates were also similar). Effect of CZP treatment was similar regardless of prior anti-TNF use, disease duration and concomitant DMARDs, based on ACR20 response rates. The percentage of patients achieving DAS28(ESR) LDA at week 28 was calculated for DAS28(ESR), SJC or CDAI responders at earlier time points. Reductions from baseline (Δ) of DAS28(ESR) <1.2, ΔSJC <25% or ΔCDAI <10 by week 12 were associated with <9% chance of achieving LDA at week 28 regardless of prior anti-TNF exposure. Adverse event rates were similar for placebo → CZP and CZP → CZP patients, with no new safety signals identified.
Conclusions: A diverse population of RA patients with varying disease duration showed rapid and sustained clinical improvements on CZP treatment, regardless of prior anti-TNF or concomitant DMARD use. Failure to achieve improvements in DAS28(ESR), SJC or CDAI within the first 12 weeks of CZP therapy was associated with a low chance of achieving LDA at week 28. No new safety signals were observed.
Trial registration: ClinicalTrials.gov, NCT00717236 , 15 July 2008.
Figures
References
-
- Sokka T, Pincus T. Most patients receiving routine care for rheumatoid arthritis in 2001 did not meet inclusion criteria for most recent clinical trials or American College of Rheumatology criteria for remission. J Rheumatol. 2003;30:1138–46. - PubMed
-
- Keystone EC, Genovese MC, Klareskog L, Hsia EC, Hall ST, Miranda PC, et al. Golimumab, a human antibody to tumour necrosis factor {alpha} given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD Study. Ann Rheum Dis. 2009;68:789–96. doi: 10.1136/ard.2008.099010. - DOI - PMC - PubMed
-
- Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37. doi: 10.1002/art.21519. - DOI - PubMed
-
- Klareskog L, van der Heijde D, de Jager JP, Gough A, Kalden J, Malaise M, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675–81. doi: 10.1016/S0140-6736(04)15640-7. - DOI - PubMed
-
- Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594–602. doi: 10.1056/NEJM200011303432202. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

