Effect of Human Adipose Tissue Mesenchymal Stem Cells on the Regeneration of Ovine Articular Cartilage
- PMID: 26569221
- PMCID: PMC4661848
- DOI: 10.3390/ijms161125989
Effect of Human Adipose Tissue Mesenchymal Stem Cells on the Regeneration of Ovine Articular Cartilage
Abstract
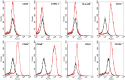
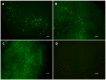
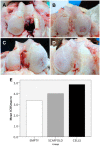
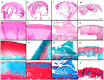
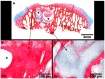
Cell therapy is a promising approach to improve cartilage healing. Adipose tissue is an abundant and readily accessible cell source. Previous studies have demonstrated good cartilage repair results with adipose tissue mesenchymal stem cells in small animal experiments. This study aimed to examine these cells in a large animal model. Thirty knees of adult sheep were randomly allocated to three treatment groups: CELLS (scaffold seeded with human adipose tissue mesenchymal stem cells), SCAFFOLD (scaffold without cells), or EMPTY (untreated lesions). A partial thickness defect was created in the medial femoral condyle. After six months, the knees were examined according to an adaptation of the International Cartilage Repair Society (ICRS 1) score, in addition to a new Partial Thickness Model scale and the ICRS macroscopic score. All of the animals completed the follow-up period. The CELLS group presented with the highest ICRS 1 score (8.3 ± 3.1), followed by the SCAFFOLD group (5.6 ± 2.2) and the EMPTY group (5.2 ± 2.4) (p = 0.033). Other scores were not significantly different. These results suggest that human adipose tissue mesenchymal stem cells promoted satisfactory cartilage repair in the ovine model.
Keywords: adult stem cell; biomaterials; cartilage; regenerative medicine; stem cell therapy; tissue engineering.
Figures



References
-
- Widuchowski W., Widuchowski J., Faltus R., Lukasik P., Kwiatkowski G., Szyluk K., Koczy B. Long-term clinical and radiological assessment of untreated severe cartilage damage in the knee: A natural history study. Scand. J. Med. Sci. Sports. 2011;21:106–110. doi: 10.1111/j.1600-0838.2009.01062.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

