The Chronic Care for Wet Age Related Macular Degeneration (CHARMED) Study: A Randomized Controlled Trial
- PMID: 26569501
- PMCID: PMC4646575
- DOI: 10.1371/journal.pone.0143085
The Chronic Care for Wet Age Related Macular Degeneration (CHARMED) Study: A Randomized Controlled Trial
Abstract
Background: In real life, outcomes in wet age related macular degeneration (W-AMD) continue to fall behind the results from randomized controlled trials. The aim of this trial was to assess if outcomes can be improved by an intervention in healthcare organization following recommendations of the Chronic Care Model (CCM).
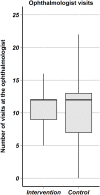
Methods: Multi-centered randomized controlled clinical trial. The multifaceted intervention consisted in reorganization of care (delivery by trained chronic care coaches, using reminder systems, performing structured follow-up, empowering patients in self-monitoring and giving decision-support). In the control usual care was continued. Main outcome measures were changes in ETDRS visual acuity, optical coherence tomography (OCT) macular retinal thickness and quality of life (NEI VFQ-25 questionnaire).
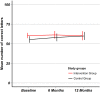
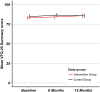
Results: 169 consecutive patients in Swiss ophthalmology centers were included. Mean ETDRS baseline visual acuity of eyes with W-AMD was 57.8 (± 18.7). After 12 months, the between-group difference in mean change of ETDRS visual acuity was -4.8 (95%CI: -10.8 to +1.2, p = 0.15); difference in mean change of OCT was +14.0 (95% CI -39.6 to 67.6, p = 0.60); difference in mean change of NEI VFQ-25 composite score mean change was +2.1(95%CI: -1.3 to +5.5, p = 0.19).
Conclusions: The intervention aiming at improving chronic care was not associated with favorable outcomes within 12 months. Other approaches need to be tested to close the evidence-performance gap in W-AMD.
Trial registration: Controlled-Trials.com ISRCTN32507927.
Conflict of interest statement
Figures

References
-
- Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA. 2004. April 21;291(15):1900–1. . - PubMed
-
- Friedman DS, O'Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004. April;122(4):564–72. . - PubMed
-
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006. October 5;355(14):1419–31. . - PubMed
-
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006. October 5;355(14):1432–44. . Epub 2006/10/06. eng. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

