Essential Role of GATA2 in the Negative Regulation of Type 2 Deiodinase Gene by Liganded Thyroid Hormone Receptor β2 in Thyrotroph
- PMID: 26571013
- PMCID: PMC4646574
- DOI: 10.1371/journal.pone.0142400
Essential Role of GATA2 in the Negative Regulation of Type 2 Deiodinase Gene by Liganded Thyroid Hormone Receptor β2 in Thyrotroph
Abstract
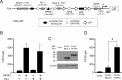
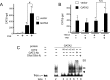
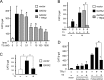
The inhibition of thyrotropin (thyroid stimulating hormone; TSH) by thyroid hormone (T3) and its receptor (TR) is the central mechanism of the hypothalamus-pituitary-thyroid axis. Two transcription factors, GATA2 and Pit-1, determine thyrotroph differentiation and maintain the expression of the β subunit of TSH (TSHβ). We previously reported that T3-dependent repression of the TSHβ gene is mediated by GATA2 but not by the reported negative T3-responsive element (nTRE). In thyrotrophs, T3 also represses mRNA of the type-2 deiodinase (D2) gene, where no nTRE has been identified. Here, the human D2 promoter fused to the CAT or modified Renilla luciferase gene was co-transfected with Pit-1 and/or GATA2 expression plasmids into cell lines including CV1 and thyrotroph-derived TαT1. GATA2 but not Pit-1 activated the D2 promoter. Two GATA responsive elements (GATA-REs) were identified close to cAMP responsive element. The protein kinase A activator, forskolin, synergistically enhanced GATA2-dependent activity. Gel-shift and chromatin immunoprecipitation assays with TαT1 cells indicated that GATA2 binds to these GATA-REs. T3 repressed the GATA2-induced activity of the D2 promoter in the presence of the pituitary-specific TR, TRβ2. The inhibition by T3-bound TRβ2 was dominant over the synergism between GATA2 and forskolin. The D2 promoter is also stimulated by GATA4, the major GATA in cardiomyocytes, and this activity was repressed by T3 in the presence of TRα1. These data indicate that the GATA-induced activity of the D2 promoter is suppressed by T3-bound TRs via a tethering mechanism, as in the case of the TSHβ gene.
Conflict of interest statement
Figures




Similar articles
-
G ATA2 mediates the negative regulation of the prepro-thyrotropin-releasing hormone gene by liganded T3 receptor β2 in the rat hypothalamic paraventricular nucleus.PLoS One. 2020 Nov 17;15(11):e0242380. doi: 10.1371/journal.pone.0242380. eCollection 2020. PLoS One. 2020. PMID: 33201916 Free PMC article.
-
Liganded T3 receptor β2 inhibits the positive feedback autoregulation of the gene for GATA2, a transcription factor critical for thyrotropin production.PLoS One. 2020 Jan 15;15(1):e0227646. doi: 10.1371/journal.pone.0227646. eCollection 2020. PLoS One. 2020. PMID: 31940421 Free PMC article.
-
Dissecting the Relation between a nuclear receptor and GATA: binding affinity studies of thyroid hormone receptor and GATA2 on TSHβ promoter.PLoS One. 2010 Sep 7;5(9):e12628. doi: 10.1371/journal.pone.0012628. PLoS One. 2010. PMID: 20838640 Free PMC article.
-
The Mechanism of Negative Transcriptional Regulation by Thyroid Hormone: Lessons From the Thyrotropin β Subunit Gene.Vitam Horm. 2018;106:97-127. doi: 10.1016/bs.vh.2017.06.006. Epub 2017 Aug 18. Vitam Horm. 2018. PMID: 29407449 Review.
-
Functional neuroanatomy of thyroid hormone feedback in the human hypothalamus and pituitary gland.Mol Cell Endocrinol. 2006 Jun 7;251(1-2):1-8. doi: 10.1016/j.mce.2006.03.042. Epub 2006 May 16. Mol Cell Endocrinol. 2006. PMID: 16707210 Review.
Cited by
-
Hematopoietic Differentiation of Human Pluripotent Stem Cells: HOX and GATA Transcription Factors as Master Regulators.Curr Genomics. 2019 Sep;20(6):438-452. doi: 10.2174/1389202920666191017163837. Curr Genomics. 2019. PMID: 32194342 Free PMC article. Review.
-
Genomic and Non-Genomic Mechanisms of Action of Thyroid Hormones and Their Catabolite 3,5-Diiodo-L-Thyronine in Mammals.Int J Mol Sci. 2020 Jun 10;21(11):4140. doi: 10.3390/ijms21114140. Int J Mol Sci. 2020. PMID: 32532017 Free PMC article. Review.
-
Nuclear Receptors and the Hidden Language of the Metabolome.Cells. 2024 Jul 31;13(15):1284. doi: 10.3390/cells13151284. Cells. 2024. PMID: 39120315 Free PMC article. Review.
-
Advances in TRH signaling.Rev Endocr Metab Disord. 2016 Dec;17(4):545-558. doi: 10.1007/s11154-016-9375-y. Rev Endocr Metab Disord. 2016. PMID: 27515033 Review.
-
G ATA2 mediates the negative regulation of the prepro-thyrotropin-releasing hormone gene by liganded T3 receptor β2 in the rat hypothalamic paraventricular nucleus.PLoS One. 2020 Nov 17;15(11):e0242380. doi: 10.1371/journal.pone.0242380. eCollection 2020. PLoS One. 2020. PMID: 33201916 Free PMC article.
References
-
- Sasaki S, Matsushita A, Nakamura H (2011) Negative regulation of the thyrotropin beta gene by thyroid hormone. Contemporary Aspects of Endocrinology, InTech http://www.intechopen.com/books/contemporary-aspects-of-endocrinology: pp. 101–138.
-
- Bianco AC, Kim BW (2012) Intracellular pathway of iodothyronine metabolism/implicatins of deiodination for thyroid hormone action. Werner & Ingbar's The Thyroid: A Fundamental and Clinical Text 10th Edition: 103–126.
-
- Koenig RJ, Leonard JL, Senator D, Rappaport N, Watson AY, Larsen PR (1984) Regulation of thyroxine 5'-deiodinase activity by 3,5,3'-triiodothyronine in cultured rat anterior pituitary cells. Endocrinology 115: 324–329. - PubMed
-
- Itagaki Y, Yoshida K, Ikeda H, Kaise K, Kaise N, Yamamoto M, et al. (1990) Thyroxine 5'-deiodinase in human anterior pituitary tumors. J Clin Endocrinol Metab 71: 340–344. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

