Re-membering the body: applications of computational neuroscience to the top-down control of regeneration of limbs and other complex organs
- PMID: 26571046
- PMCID: PMC4667987
- DOI: 10.1039/c5ib00221d
Re-membering the body: applications of computational neuroscience to the top-down control of regeneration of limbs and other complex organs
Abstract
A major goal of regenerative medicine and bioengineering is the regeneration of complex organs, such as limbs, and the capability to create artificial constructs (so-called biobots) with defined morphologies and robust self-repair capabilities. Developmental biology presents remarkable examples of systems that self-assemble and regenerate complex structures toward their correct shape despite significant perturbations. A fundamental challenge is to translate progress in molecular genetics into control of large-scale organismal anatomy, and the field is still searching for an appropriate theoretical paradigm for facilitating control of pattern homeostasis. However, computational neuroscience provides many examples in which cell networks - brains - store memories (e.g., of geometric configurations, rules, and patterns) and coordinate their activity towards proximal and distant goals. In this Perspective, we propose that programming large-scale morphogenesis requires exploiting the information processing by which cellular structures work toward specific shapes. In non-neural cells, as in the brain, bioelectric signaling implements information processing, decision-making, and memory in regulating pattern and its remodeling. Thus, approaches used in computational neuroscience to understand goal-seeking neural systems offer a toolbox of techniques to model and control regenerative pattern formation. Here, we review recent data on developmental bioelectricity as a regulator of patterning, and propose that target morphology could be encoded within tissues as a kind of memory, using the same molecular mechanisms and algorithms so successfully exploited by the brain. We highlight the next steps of an unconventional research program, which may allow top-down control of growth and form for numerous applications in regenerative medicine and synthetic bioengineering.
Figures



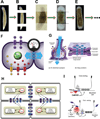
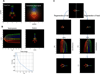



References
-
- Ingber DE, Levin M. What lies at the interface of regenerative medicine and developmental biology? Development. 2007;134:2541–2547. - PubMed
- Stoick-Cooper CL, Moon RT, Weidinger G. Advances in signaling in vertebrate regeneration as a prelude to regenerative medicine. Genes Dev. 2007;21:1292–1315. - PubMed
-
- French V. Positional information around the segments of the cockroach leg. Journal of embryology and experimental morphology. 1980;59:281–313. - PubMed
-
- Oviedo NJ, Newmark PA, Sanchez Alvarado A. Allometric scaling and proportion regulation in the freshwater planarian Schmidtea mediterranea . Dev Dyn. 2003;226:326–333. - PubMed
-
- Farinella-Ferruzza N. The transformation of a tail into a limb after xenoplastic transformation. Experientia. 1956;15:304–305.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

