Rhinovirus Detection in Symptomatic and Asymptomatic Children: Value of Host Transcriptome Analysis
- PMID: 26571305
- PMCID: PMC4824929
- DOI: 10.1164/rccm.201504-0749OC
Rhinovirus Detection in Symptomatic and Asymptomatic Children: Value of Host Transcriptome Analysis
Abstract
Rationale: Rhinoviruses (RVs) are a major cause of symptomatic respiratory tract infection in all age groups. However, RVs can frequently be detected in asymptomatic individuals.
Objectives: To evaluate the ability of host transcriptional profiling to differentiate between symptomatic RV infection and incidental detection in children.
Methods: Previously healthy children younger than 2 years old (n = 151) were enrolled at four study sites and classified into four clinical groups: RV- healthy control subjects (n = 37), RV+ asymptomatic subjects (n = 14), RV+ outpatients (n = 30), and RV+ inpatients (n = 70). Host responses were analyzed using whole-blood RNA transcriptional profiles.
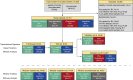
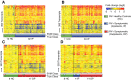
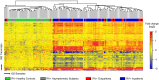
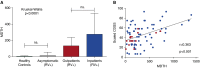
Measurements and main results: RV infection induced a robust transcriptional signature, which was validated in three independent cohorts and by quantitative real-time polymerase chain reaction with high prediction accuracy. The immune profile of symptomatic RV infection was characterized by overexpression of innate immunity and underexpression of adaptive immunity genes, whereas negligible changes were observed in asymptomatic RV+ subjects. Unsupervised hierarchical clustering identified two main clusters of subjects. The first included 93% of healthy control subjects and 100% of asymptomatic RV+ subjects, and the second comprised 98% of RV+ inpatients and 88% of RV+ outpatients. Genomic scores of healthy control subjects and asymptomatic RV+ children were similar and significantly lower than those of RV+ inpatients and outpatients (P < 0.0001).
Conclusions: Symptomatic RV infection induced a robust and reproducible transcriptional signature, whereas identification of RV in asymptomatic children was not associated with significant systemic transcriptional immune responses. Transcriptional profiling represents a useful tool to discriminate between active infection and incidental virus detection.
Keywords: asymptomatic; children; rhinovirus; transcriptional profiling; viral detection.
Figures


Comment in
-
An Expression of Clinical Significance: Exploring the Human Genome to Understand the Variable Response to Rhinovirus.Am J Respir Crit Care Med. 2016 Apr 1;193(7):710-2. doi: 10.1164/rccm.201511-2272ED. Am J Respir Crit Care Med. 2016. PMID: 27035780 Free PMC article. No abstract available.
References
-
- Iwane MK, Prill MM, Lu X, Miller EK, Edwards KM, Hall CB, Griffin MR, Staat MA, Anderson LJ, Williams JV, et al. Human rhinovirus species associated with hospitalizations for acute respiratory illness in young US children. J Infect Dis. 2011;204:1702–1710. - PubMed
-
- Rakes GP, Arruda E, Ingram JM, Hoover GE, Zambrano JC, Hayden FG, Platts-Mills TA, Heymann PW. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care: IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999;159:785–790. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

