Mesodermal iPSC-derived progenitor cells functionally regenerate cardiac and skeletal muscle
- PMID: 26571398
- PMCID: PMC4665797
- DOI: 10.1172/JCI82735
Mesodermal iPSC-derived progenitor cells functionally regenerate cardiac and skeletal muscle
Abstract
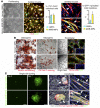
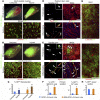
Conditions such as muscular dystrophies (MDs) that affect both cardiac and skeletal muscles would benefit from therapeutic strategies that enable regeneration of both of these striated muscle types. Protocols have been developed to promote induced pluripotent stem cells (iPSCs) to differentiate toward cardiac or skeletal muscle; however, there are currently no strategies to simultaneously target both muscle types. Tissues exhibit specific epigenetic alterations; therefore, source-related lineage biases have the potential to improve iPSC-driven multilineage differentiation. Here, we determined that differential myogenic propensity influences the commitment of isogenic iPSCs and a specifically isolated pool of mesodermal iPSC-derived progenitors (MiPs) toward the striated muscle lineages. Differential myogenic propensity did not influence pluripotency, but did selectively enhance chimerism of MiP-derived tissue in both fetal and adult skeletal muscle. When injected into dystrophic mice, MiPs engrafted and repaired both skeletal and cardiac muscle, reducing functional defects. Similarly, engraftment into dystrophic mice of canine MiPs from dystrophic dogs that had undergone TALEN-mediated correction of the MD-associated mutation also resulted in functional striatal muscle regeneration. Moreover, human MiPs exhibited the same capacity for the dual differentiation observed in murine and canine MiPs. The findings of this study suggest that MiPs should be further explored for combined therapy of cardiac and skeletal muscles.
Figures










Comment in
-
Memory or amnesia: the dilemma of stem cell therapy in muscular dystrophies.J Clin Invest. 2015 Dec;125(12):4331-3. doi: 10.1172/JCI85002. Epub 2015 Nov 16. J Clin Invest. 2015. PMID: 26571391 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

