A collagen VI-dependent pathogenic mechanism for Hirschsprung's disease
- PMID: 26571399
- PMCID: PMC4665793
- DOI: 10.1172/JCI83178
A collagen VI-dependent pathogenic mechanism for Hirschsprung's disease
Abstract
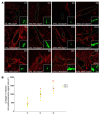
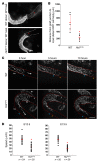
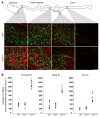
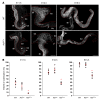
Hirschsprung's disease (HSCR) is a severe congenital anomaly of the enteric nervous system (ENS) characterized by functional intestinal obstruction due to a lack of intrinsic innervation in the distal bowel. Distal innervation deficiency results from incomplete colonization of the bowel by enteric neural crest cells (eNCCs), the ENS precursors. Here, we report the generation of a mouse model for HSCR--named Holstein--that contains an untargeted transgenic insertion upstream of the collagen-6α4 (Col6a4) gene. This insertion induces eNCC-specific upregulation of Col6a4 expression that increases total collagen VI protein levels in the extracellular matrix (ECM) surrounding both the developing and the postnatal ENS. Increased collagen VI levels during development mainly result in slower migration of eNCCs. This appears to be due to the fact that collagen VI is a poor substratum for supporting eNCC migration and can even interfere with the migration-promoting effects of fibronectin. Importantly, for a majority of patients in a HSCR cohort, the myenteric ganglia from the ganglionated region are also specifically surrounded by abundant collagen VI microfibrils, an outcome accentuated by Down syndrome. Collectively, our data thus unveil a clinically relevant pathogenic mechanism for HSCR that involves cell-autonomous changes in ECM composition surrounding eNCCs. Moreover, as COL6A1 and COL6A2 are on human Chr.21q, this mechanism is highly relevant to the predisposition of patients with Down syndrome to HSCR.
Figures



Comment in
-
Hirschsprung's disease, Down syndrome, and missing heritability: too much collagen slows migration.J Clin Invest. 2015 Dec;125(12):4323-6. doi: 10.1172/JCI85003. Epub 2015 Nov 16. J Clin Invest. 2015. PMID: 26571392 Free PMC article.
-
Motility: Hirschsprung disease--laying down a suitable path.Nat Rev Gastroenterol Hepatol. 2016 Jan;13(1):7-8. doi: 10.1038/nrgastro.2015.213. Nat Rev Gastroenterol Hepatol. 2016. PMID: 26701374 No abstract available.
References
-
- Obermayr F, Hotta R, Enomoto H, Young HM. Development and developmental disorders of the enteric nervous system. Nat Rev Gastroenterol Hepatol. 2013;10(1):43–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

